Rationale and Objectives
The aim of this study was to evaluate the intratumoral residence time of microbubbles (MBs) targeted to α v β 3 integrin expressed in the endothelial cells of mice during the process of tumor angiogenesis.
Materials and Methods
For the preparation of MBs, decafluorobutane gas was sonically dispersed in phosphate buffer saline containing L-A-phosphatidylcholine-distearoyl, polyethylene glycol 40 stearate, and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[biotinyl(polyethylene glycol)2000] in a 77:15:8 molar ratio. Avidin–fluorescein isothiocyanate and biotin–cyclic arginine-glycine-aspartate-D-tyrosine-lysine (cRGD) or biotin–alanine-glycine-aspartate (AGD) conjugates were added to the reaction mixture. Adhesion testing of the targeting MBs was performed for the MS-1 cell line expressing α v β 3 integrin in vitro. The in vivo acoustic properties of the MBs were assessed by clinical ultrasound on the HT1080 fibrosarcoma model ( n = 8) for 1 hour. Cryosections were stained with hematoxylin and eosin and by immunohistochemical staining to identify expression of α v β 3 integrin in the HT1080 tumor.
Results
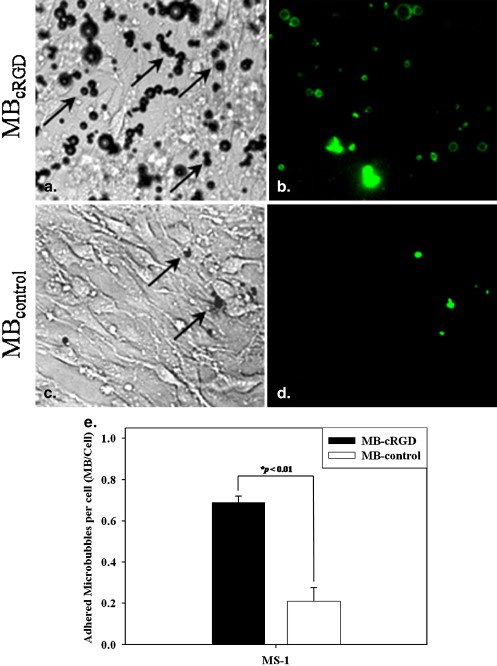
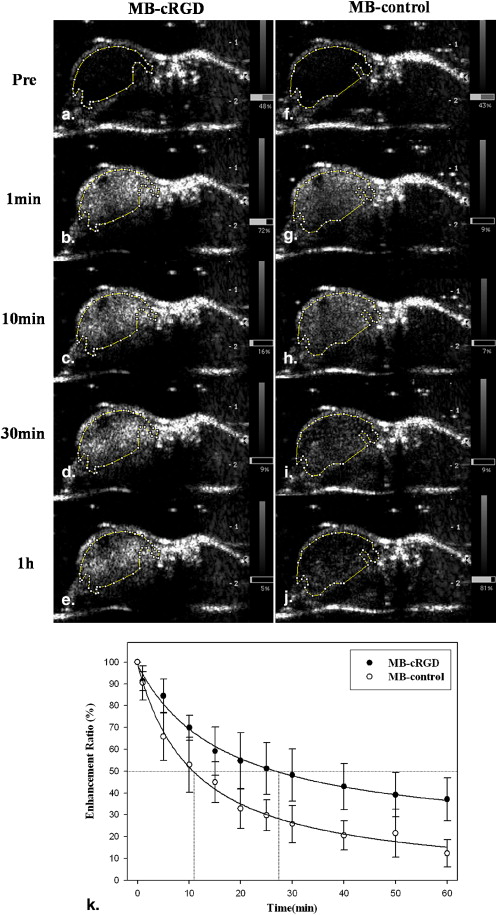
The adherence of the MBs conjugated to cRGD was significantly greater than the adherence of the MBs conjugated to biotin-AGD ( P < .01) for the MS-1 endothelial cell line. The acoustic enhancement on ultrasound was observed as a stable imaging window until 1 hour after injection of the MB conjugates in the mice. The MBs targeted via cRGD preferentially adhered to the vascular endothelium of the HT-1080 tumors. The findings of ultrasound imaging were correlated with immunohistochemical findings for the expression of α v β 3 integrin on the vascular endothelium of the tumors.
Conclusions
The prepared MBs conjugated with cRGD demonstrated a sufficient residence time to attach to the target integrin of tumor tissues. This finding suggests that the MBs are a potential molecular contrast agent that enables characterization of tumor angiogenesis and the monitoring of antitumor and antiangiogenic therapy.
Contrast-enhanced ultrasound is a recently developed imaging technique in which microbubbles (MBs) enable improved contrast between blood vessels and the surrounding tissue during ultrasound imaging. Several studies have recently been performed for MB-specific applications, including tissue-specific reticuloendothelial system imaging , molecular targeting imaging , drug delivery and gene therapy . Several academic and industrial research groups with different animal models have shown the successful use of targeted ultrasound contrast agents for qualitative ultrasound-based imaging.
Angiogenesis is a critical determinant of tumor growth, invasion, and metastatic potential. Specific endothelial molecular markers of angiogenesis are expressed in the tumor vasculature. Alpha v β 3 integrin is well known as an adhesion protein, which is upregulated in activated endothelial cells such as the cells in the neovasculature within ischemic tissue and tumors. Therefore, detection of α v β 3 integrin expression in tumor neovessels may be useful because this integrin appears to have a functional role in angiogenesis , and it has been implicated as a marker of the metastatic potential and of poor prognosis . Some studies have reported that tumor angiogenesis could be assessed with the use of ultrasound imaging and contrast agents targeted to integrins .
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Preparation of MBs
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
In Vitro Adhesion Test of MB-cRGD to Endothelial Cells
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Murine Tumor Model
Get Radiology Tree app to read full this article<
Contrast-enhanced Ultrasound Imaging
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Histologic Confirmation of in Vivo MB-cRGD Binding
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Characterization of the MBs
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
In Vitro Adhesion Test of MB-cRGD to Endothelial Cells
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Contrast-Enhanced Ultrasound Imaging
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Quantitative Data of Video Intensity for Contrast-enhanced Ultrasound Images Following MB Administration in HT1080 Tumor Model
MB-Control MB-cRGD Imaging Time After Injection ( n = 8) ( n = 8) Before contrast 24.57 ± 4.72 22.49 ± 8.47 10 s 77.96 ± 17.63 72.77 ± 17.22 1 min 72.27 ± 14.94 68.07 ± 15.33 10 min 52.66 ± 12.55 56.63 ± 12.73 30 min 39.86 ± 12.52 47.11 ± 12.68 60 min 30.74 ± 8.24 43.30 ± 13.28
MB, microbubble; MB-control, microbubbles conjugated with biotin–alanine-glycine-aspartate; MB-cRGD, microbubbles conjugated with cyclic arginine-glycine-aspartate-D-tyrosine-lysine.
Data are expressed as mean ± standard deviation.
Table 2
Values of the Contrast Enhancement Ratio for Contrast-enhanced Ultrasound Images Following MB Administration in HT1080 Tumor Model
Imaging Time After Injection (min) MB-Control (%) MB-cRGD (%)P Before contrast 100 100 — 1 90.35 ± 7.17 90.56 ± 4.50 .083 10 53.39 ± 11.58 66.27 ± 10.92 .149 20 32.92 ± 8.31 53.95 ± 11.97 .083 30 27.93 ± 9.65 47.85 ± 10.97 .043 ∗ 40 20.92 ± 6.17 42.85 ± 9.64 .043 ∗ 50 18.34 ± 8.10 39.63 ± 9.29 .043 ∗ 60 11.38 ± 6.34 37.54 ± 9.12 .043 ∗
MB, microbubble; MB-control, microbubbles conjugated with biotin–alanine-glycine-aspartate; MB-cRGD, microbubbles conjugated with cyclic arginine-glycine-aspartate-D-tyrosine-lysine.
Data are expressed as mean ± standard deviation.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Histologic Confirmation of in Vivo MB-cRGD Binding
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Acknowledgments
Get Radiology Tree app to read full this article<
References
1. Kono Y., Steinbach G.C., Peterson T., et. al.: Mechanism of parenchymal enhancement of the liver with a microbubble-based US contrast medium: an intravital microscopy study in rats. Radiology 2002; 224: pp. 253-257.
2. Leong-Poi H., Christiansen J., Klibanov A.L., et. al.: Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to alpha(v)-integrins. Circulation 2003; 107: pp. 455-460.
3. Lum A.F., Borden M.A., Dayton P.A., et. al.: Ultrasound radiation force enables targeted deposition of model drug carriers loaded on microbubbles. J Control Release 2006; 111: pp. 128-134.
4. Klibanov A.L.: Microbubble contrast agents: targeted ultrasound imaging and ultrasound-assisted drug-delivery applications. Invest Radiol 2006; 41: pp. 354-362.
5. Borden M.A., Caskey C.F., Little E., et. al.: DNA and polylysine adsorption and multilayer construction onto cationic lipid-coated microbubbles. Langmuir 2007; 23: pp. 9401-9408.
6. Leong-Poi H., Kuliszewski M.A., Lekas M., et. al.: Therapeutic arteriogenesis by ultrasound-mediated VEGF165 plasmid gene delivery to chronically ischemic skeletal muscle. Circ Res 2007; 101: pp. 295-303.
7. Suzuki R., Takizawa T., Negishi Y., et. al.: Effective gene delivery with liposomal bubbles and ultrasound as novel non-viral system. J Drug Target 2007; 15: pp. 531-537.
8. Brooks P.C., Clark R.A., Cheresh D.A.: Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994; 264: pp. 569-571.
9. Sipkins D.A., Cheresh D.A., Kazemi M.R., et. al.: Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat Med 1998; 4: pp. 623-626.
10. Gasparini G., Brooks P.C., Biganzoli E., et. al.: Vascular integrin alpha(v)beta3: a new prognostic indicator in breast cancer. Clin Cancer Res 1998; 4: pp. 2625-2634.
11. Weller G.E., Wong M.K., Modzelewski R.A., et. al.: Ultrasonic imaging of tumor angiogenesis using contrast microbubbles targeted via the tumor-binding peptide arginine-arginine-leucine. Cancer Res 2005; 65: pp. 533-539.
12. Ellegala D.B., Leong-Poi H., Carpenter J.E., et. al.: Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v)beta3. Circulation 2003; 108: pp. 336-341.
13. Hasik M.J., Kim D.H., Howle L.E., et. al.: Evaluation of synthetic phospholipid ultrasound contrast agents. Ultrasonics 2002; 40: pp. 973-982.
14. Haubner R., Wester H.J., Reuning U., et. al.: Radiolabeled alpha(v)beta3 integrin antagonists: a new class of tracers for tumor targeting. J Nucl Med 1999; 40: pp. 1061-1071.
15. Thumshirn G., Hersel U., Goodman S.L., et. al.: Multimeric cyclic RGD peptides as potential tools for tumor targeting: solid-phase peptide synthesis and chemoselective oxime ligation. Chemistry 2003; 9: pp. 2717-2725.
16. Klibanov A.L.: Ligand-carrying gas-filled microbubbles: ultrasound contrast agents for targeted molecular imaging. Bioconj Chem 2005; 16: pp. 9-17.
17. Joseph S., Olbrich C., Kirsch J., et. al.: A real-time in vitro assay for studying functional characteristics of target-specific ultrasound contrast agents. Pharm Res 2004; 21: pp. 920-926.
18. Choi K.S., Kim S.H., Cai Q.Y., et. al.: Inflammation-specific T1 imaging using anti-intercellular adhesion molecule 1 antibody-conjugated gadolinium diethylenetriaminepentaacetic acid. Mol Imaging 2007; 6: pp. 75-84.
19. Klibanov A.L., Rychak J.J., Yang W.C., et. al.: Targeted ultrasound contrast agent for molecular imaging of inflammation in high-shear flow. Contrast Media Mol Imaging 2006; 1: pp. 259-266.
20. Ferrara K., Pollard R., Borden M.: Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng 2007; 9: pp. 415-447.
21. Lindner J.R., Song J., Christiansen J., et. al.: Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation 2001; 104: pp. 2107-2112.
22. Janssen M.L., Oyen W.J., Dijkgraaf I., et. al.: Tumor targeting with radiolabeled alpha(v)beta(3) integrin binding peptides in a nude mouse model. Cancer Res 2002; 62: pp. 6146-6151.
![Figure 4, Fluorescence microscopic images show the fluorescent integrin (cyclic arginine-glycine-aspartate-D-tyrosine-lysine [cRGD]) signal on the endothelium of the tumor vessels on the HT1080 tumor (a,b) . The images show that the amount of the fluorescent signal for microbubbles (MBs) conjugated with cRGD (MB-cRGD) (b) was significantly greater than that for MBs conjugated with biotin–alanine-glycine-aspartate (MB-control) (a) on the tumor. (c,d) Hematoxylin and eosin staining and immunohistochemical staining of α v β 3 -integrin of the HT1080 tumor. The α v (c) and β 3 (d) integrin is expressed on the tumor vessels of the tumor.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/LongResidenceTimeofUltrasoundMicrobubblesTargetedtoIntegrininMurineTumorModel/3_1s20S1076633209004152.jpg)