Rationale and Objectives
The purpose of this study was to evaluate the relative merits of micro-computed tomograph colonography (mCTC) and optical colonoscopy (OC) for longitudinal studies of colonic tumors in mice.
Materials and Methods
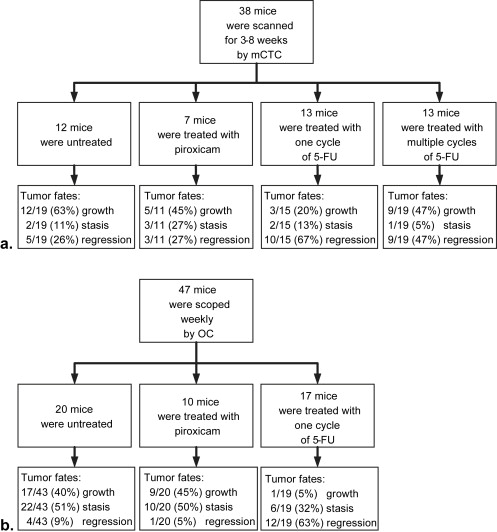
Colonic tumors in mice carrying the Min allele of Apc were followed over several weeks using mCTC and OC. A total of 146 colonic tumors were monitored: 62 in 32 untreated Min mice, 53 in 43 Min mice treated with 5-fluorouracil (5-FU), and 31 in 17 Min mice treated with piroxicam.
Results
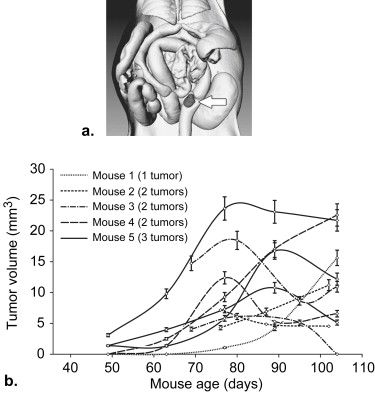
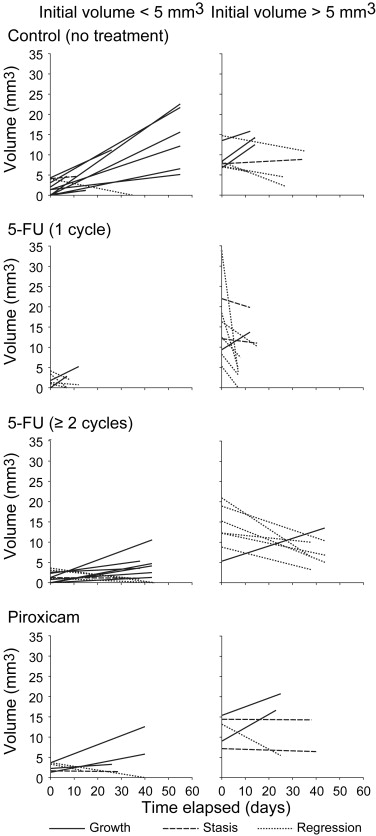
Colonic tumors in Min mice had three different spontaneous fates: 29 grew, 24 remained static, and 9 regressed. Treating Min mice with 5-FU increased the percentage of regressing tumors from 15% to 58%. The response was dependent in part on the initial size of the tumor. By contrast, treating Min mice with piroxicam did not alter colonic tumor fate.
Conclusions
mCTC and OC can be used to determine the spontaneous fates of colonic tumors in mice and to document their individual responses to treatment. The ability to follow individually annotated colonic tumors reduces the number of mice needed for testing.
Murine models of human colorectal cancer afford investigators the opportunity to rapidly test newly developed treatments. The Apc Min/+ (Min) mouse carries a nonsense mutation in codon 850 of the Adenomatous polyposis coli ( Apc ) gene and develops benign adenomas along the entire length of the intestinal tract . This model has been used over the past 18 years to test at least 269 treatments, including dietary supplements, nonsteroidal anti-inflammatory drugs, and exercise . For example, Jacoby et al exposed 15 Min mice to 100 ppm piroxicam via the diet for 42 days . Tumor multiplicity in a 4-cm segment of the distal small intestine was reduced from 10 ± 2 to 2 ± 1. This protective effect of piroxicam in the small intestine has been confirmed in several independent studies .
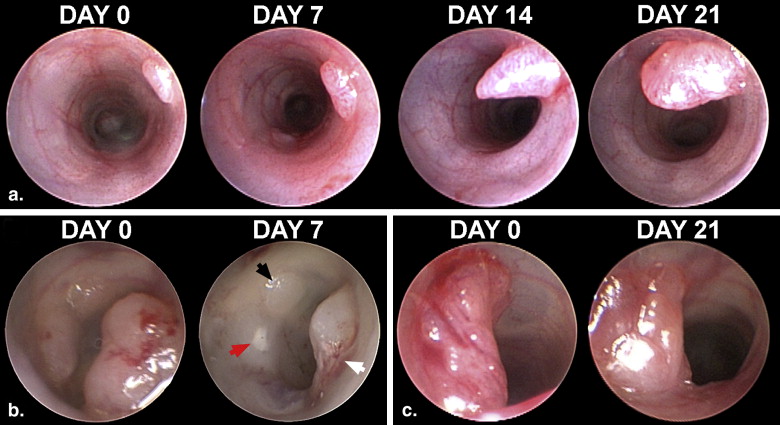
A limitation of genetic mouse models for human colon cancer is that tumors develop primarily in the small intestine instead of the colon . The low multiplicity of colonic tumors leads to the need for large experimental cohorts of mice for statistical significance in studies with a traditional cross-sectional design. This limitation can be overcome by longitudinally monitoring individually annotated colonic tumors in mice, where the statistical power depends on the ability to monitor the tumor response rather than on tumor multiplicity. In vivo imaging modalities such as magnetic resonance imaging (MRI), micro-computed tomograph colonography (mCTC), and optical colonoscopy (OC) may provide complementary tools to monitor longitudinally the biology and therapeutics of colonic tumors .
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Methods
Mice
Get Radiology Tree app to read full this article<
Treatment with 5-FU or Piroxicam
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
MicroCT Colonography
Get Radiology Tree app to read full this article<
Multiple Regression Analysis
Get Radiology Tree app to read full this article<
Optical Colonoscopy (OC)
Get Radiology Tree app to read full this article<
Treatment with Dextran Sodium Sulfate (DSS) Plus Piroxicam
Get Radiology Tree app to read full this article<
Power
Get Radiology Tree app to read full this article<
Results
The Range of Tumor Fates in Control Mice
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Response of Colonic Tumors to 5-FU
Get Radiology Tree app to read full this article<
Table 1
Several Factors Affect Tumor Volume in Treated Mice and Controls
95% Confidence Interval Factor ∗ Mean Effect † Standard Error Lower Upper_P_ Value Initial volume ‡ -0.042 0.017 -0.080 -0.017 .014 Sex § 0.199 0.075 0.041 0.343 .008 5-FU -0.335 0.092 -0.531 -0.170 .001 Age 0.002 0.003 -0.004 0.007 .502 Body mass -0.062 0.040 -0.143 0.016 .121
5-FU, 5-fluorouracil.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Resistance of Colonic Tumors to Piroxicam
Get Radiology Tree app to read full this article<
DSS-piroxicam Treatment of Min Mice Alters Tumor Distribution and Leads to Enhanced Longevity
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Acknowledgments
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Moser A.R., Pitot H.C., Dove W.F.: A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 1990; 247: pp. 322-324.
2. Shoemaker A.R., Gould K.A., Luongo C., et. al.: Studies of neoplasia in the Min mouse. Biochim Biophys Acta 1997; 1332: pp. F25-F48.
3. Corpet D.E., Pierre F.: Point: from animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev 2003; 12: pp. 391-400.
4. Jacoby R.F., Cole C.E., Tutsch K., et. al.: Chemopreventive efficacy of combined piroxicam and difluoromethylornithine treatment of Apc mutant Min mouse adenomas, and selective toxicity against Apc mutant embryos. Cancer Res 2000; 60: pp. 1864-1870.
5. Ritland S.R., Gendler S.J.: Chemoprevention of intestinal adenomas in the Apc Min mouse by piroxicam: kinetics, strain effects and resistance to chemosuppression. Carcinogenesis 1999; 20: pp. 51-58.
6. Jacoby R.F., Marshall D.J., Newton M.A., et. al.: Chemoprevention of spontaneous intestinal adenomas in the Apc Min mouse model by the nonsteroidal anti-inflammatory drug piroxicam. Cancer Res 1996; 56: pp. 710-714.
7. Jacoby R.F., Seibert K., Cole C.E., et. al.: The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the Min mouse model of adenomatous polyposis. Cancer Res 2000; 60: pp. 5040-5044.
8. Bruce W.R.: Counterpoint: from animal models to prevention of colon cancer. Criteria for proceeding from preclinical studies and choice of models for prevention studies. Cancer Epidemiol Biomarkers Prev 2003; 12: pp. 401-404.
9. Hensley H.H., Chang W.C., Clapper M.L.: Detection and volume determination of colonic tumors in Min mice by magnetic resonance micro-imaging. Magn Reson Med 2004; 52: pp. 524-529.
10. Hensley H.H., Merkel C.E., Chang W.C., et. al.: Endoscopic imaging and size estimation of colorectal adenomas in the multiple intestinal neoplasia mouse. Gastrointest Endosc 2009; 69: pp. 742-749.
11. Pickhardt P.J., Halberg R.B., Taylor A.J., et. al.: Microcomputed tomography colonography for polyp detection in an in vivo mouse tumor model. Proc Natl Acad Sci U S A 2005; 102: pp. 3419-3422.
12. Becker C., Fantini M.C., Neurath M.F.: High resolution colonoscopy in live mice. Nature Protocols 2006; 1: pp. 2900-3904.
13. Durkee B.Y., Mudd S.R., Roen C.N., et. al.: Reproducibility of tumor volume measurement at microCT colonography in living mice. Acad Radiol 2008; 15: pp. 334-341.
14. Becker C., Fantini M.C., Wirtz S., et. al.: In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut 2005; 54: pp. 950-954.
15. Cooper H.S., Everley L., Chang W.C., et. al.: The role of mutant Apc in the development of dysplasia and cancer in the mouse model of dextran sulfate sodium-induced colitis. Gastroenterology 2001; 121: pp. 1407-1416.
16. Akaike H.: New look at statistical-model identification. IEEE Trans Automatic Control 1974; AC19: pp. 716-723.
17. Efron B., Tibshirani R.J.: An introduction to the bootstrap.1993.Chapman & Hall/CRCBoca Raton, FL
18. Ware J.H.: Linear models for the analysis of longitudinal studies. Am Statistician 1985; 39: pp. 95-101.
19. Kim D.H., Pickhardt P.J., Taylor A.J., et. al.: CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med 2007; 357: pp. 1403-1412.
20. Jackson-Thompson J., Ahmed F., German R.R., et. al.: Descriptive epidemiology of colorectal cancer in the United States, 1998–2001. Cancer 2006; 107: pp. 1103-1111.
21. Regula J., Rupinski M., Kraszewska E., et. al.: Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med 2006; 355: pp. 1863-1872.
22. Rossouw J.E., Anderson G.L., Prentice R.L., et. al.: Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288: pp. 321-333.
23. Amos-Landgraf J.M., Kwong L.N., Kendziorski C.M., et. al.: A target-selected Apc -mutant rat kindred enhances the modeling of familial human colon cancer. Proc Natl Acad Sci U S A 2007; 104: pp. 4036-4041.
24. Thirion P., Michiels S., Pignon J.P., et. al.: Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol 2004; 22: pp. 3766-3775.
25. Blijham G.H.: Chemotherapy of colorectal cancer. Anticancer Drugs 1991; 2: pp. 233-245.
26. Tucker J.M., Davis C., Kitchens M.E., et. al.: Response to 5-fluorouracil chemotherapy is modified by dietary folic acid deficiency in Apc Min/+ mice. Cancer Lett 2002; 187: pp. 153-162.
27. Uronis J.M., Herfarth H.H., Rubinas T.C., et. al.: Flat colorectal cancers are genetically determined and progress to invasion without going through a polypoid stage. Cancer Res 2007; 67: pp. 11594-11600.
28. Gambino J.J., Lindberg R.G.: Response of the pocket mouse to ionizing radiation. Radiat Res 1964; 22: pp. 586-597.
29. Halberg R.B., Waggoner J., Rasmussen K.: Long-lived Min mice develop advanced intestinal cancers through a genetically conservative pathway. Cancer Research 2009; 69: pp. 5768-5775.