Rationale and Objectives
Most secondary intramammary tumors occur as metastatic involvement from the contralateral breast. Breast metastases (BM) from nonmammary malignancies are very rare. The aims of this study were to estimate retrospectively the prevalence of BM from nonmammary malignancies and to describe their radiologic appearance.
Materials and Methods
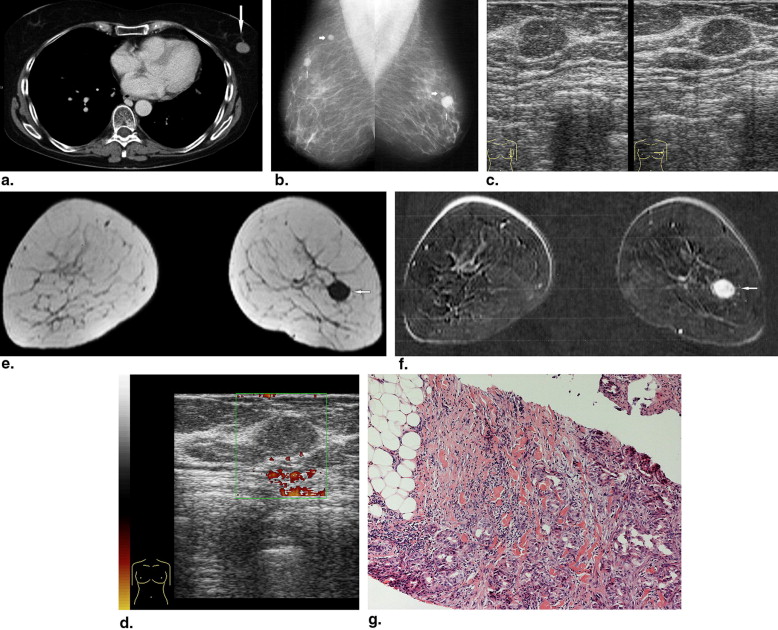
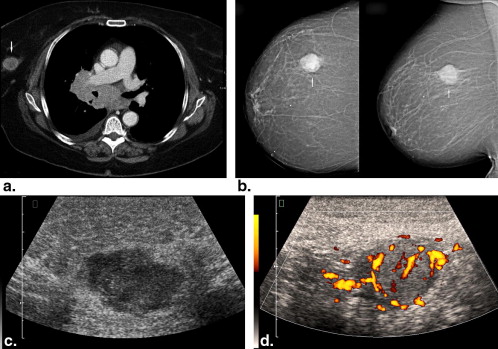
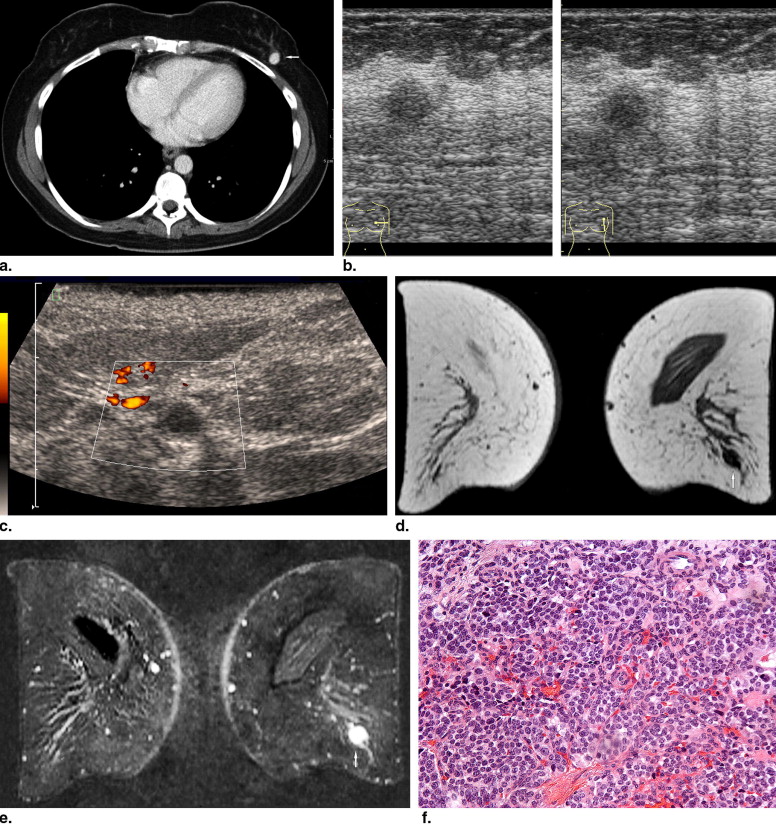
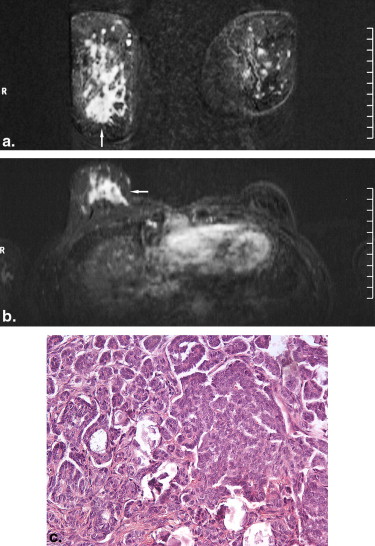
BM were identified in 51 patients, including 43 women and eight men with a median age of 61 years (range, 24–84 years). Computed tomography of the thoracic region identified 108 lesions in 38 patients. Mammography was available for 37 patients (54 lesions). Ultrasound evaluation was performed in 43 patients (71 lesions). In 24 patients (93 lesions), magnetic resonance imaging of the breast was done. Images were reviewed in consensus by two radiologists according to the Breast Imaging Reporting and Data System lexicon.
Results
The prevalence of BM in several tumors ranged from 0.12% to 4.92%. On computed tomography, most metastases were round or oval in shape with marked or moderate enhancement. On mammography, solitary or multiple round or oval masses with circumscribed margins were the most common pattern of BM. Ten percent showed microcalcifications. On ultrasound, most BM were hypoechoic, oval or round in shape, with microlobulated or circumscribed margins, and posterior acoustic enhancement. Doppler imaging showed hypervascularity in 39% of BM. On magnetic resonance imaging, most lesions demonstrated marked homogenous contrast enhancement. Type 1 kinetic curve was seen in 18%, type 2 in 52%, and type 3 in 30%.
Conclusions
The radiologic features reported in this study should be taken into consideration in the differential diagnosis of breast lesions.
Most secondary intramammary tumors occur as metastatic involvement from the contralateral breast . Breast metastases (BM) from nonmammary malignancies are very rare . This may related to the fact that the breast contains large areas of fibrous tissue with a relatively poor blood supply .
Alva and Shetty-Alva found in their review of the literature that most BM arise from malignant melanomas, sarcomas, lung cancers, ovarian tumors, renal carcinomas, and thyroid tumors in decreased order of frequency . Some single-institution studies have suggested, however, that BM from ovarian and renal carcinomas are very rare .
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Get Radiology Tree app to read full this article<
Patients
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Imaging and Review
Computed Tomography
Get Radiology Tree app to read full this article<
Mammography
Get Radiology Tree app to read full this article<
Ultrasound
Get Radiology Tree app to read full this article<
MRI
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
initial SI(%)=(SI1−3min−SIP)/SIP×100%. initial SI
(
%
)
=
(
SI
1
−
3
min
−
SI
P
)
/
SI
P
×
100
%
.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
postinitial SI(%)=(SIpeak−SIend)/SIpeak×100%. postinitial SI
(
%
)
=
(
SI
peak
−
SI
end
)
/
SI
peak
×
100
%
.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Histologic Analysis
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Primary Tumors and Number and Prevalence of BM
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Prevalence of BM in Metastasizing Oncologic Diseases
Malignant Disease Prevalence of BM (%) Malignant melanoma of skin 4.92 Carcinoma of ovary 4.43 Carcinoma of stomach 1.47 Renal cell carcinoma 1.17 Sarcoma 1.13 Carcinoma of bronchus and lung 0.89 Carcinoma of larynx 0.77 Carcinoma of cervix uteri 0.76 Seminoma of testis 0.52 Carcinoma of prostate 0.24 Carcinoma of thyroid gland 0.22 Carcinoma of colon 0.12
BM, breast metastases.
Get Radiology Tree app to read full this article<
Clinical Findings
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Radiologic Features
Computed Tomography
Get Radiology Tree app to read full this article<
Table 2
Computed Tomographic Findings of Breast Metastases
Characteristics n (%) Lesions 108 (100) Shape Oval 18 (17) Round 82 (76) Lobulated 8 (7) Margin Circumscribed 101 (94) Lobulated 7 (6) Enhancement High 56 (52) Moderate 40 (37) Low 12 (11)
Get Radiology Tree app to read full this article<
Mammography
Get Radiology Tree app to read full this article<
Table 3
Mammographic Findings in 37 Patients with Breast Metastases
Characteristic Patients Pattern Mass 27 (73%) Architectural distortion 2 (5%) No abnormalities 8 (22%) Mass features (n = 52) Lesions Shape Round 37 (71%) Oval 9 (17%) Lobular 6 (12%) Margin Circumscribed 46 (88%) Microlobulated 6 (12%) Density High 24 (46%) Isodense 28 (54%) Calcifications Yes 5 (10%) No 47 (90%) ACR 1 31% 2 31% 3 12% 4 26% BI-RADS 0 20% 3 14% 4 37% 5 29%
ACR, American College of Radiology; BI-RADS, Breast Imaging Reporting and Data System.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Ultrasound
Get Radiology Tree app to read full this article<
Table 4
Ultrasound Findings of Breast Metastases
Characteristic Patients Pattern Mass 41 (95%) Architectural distortion (diffuse infiltration) 2 (5%) Mass features (n = 68) Lesions Shape Round 30 (44%) Oval 28 (41%) Lobular 10 (15%) Margin Microlobulated 34 (50%) Circumscribed 18 (26%) Indistinct 16 (24%) Echo pattern Anechoic 13 (19%) Hypoechoic 35 (52%) Mixed hypoechoic to hyperechoic 20 (29%) Posterior phenomena Enhancement 52 (76%) None 11 (16%) Shadowing 4 (6%) Mixed 1 (2%)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 5
Doppler Findings of 42 Breast Metastases in 20 Patients
Hypervascularity n (%) None 25 (61) Light 10 (24) Moderate 2 (5) High 4 (10)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
MRI
Get Radiology Tree app to read full this article<
Table 6
Magnetic Resonance Findings of Breast Metastases
Characteristics Patients Pattern Mass 22 (92%) Diffuse infiltration 2 (8%) Mass features (n = 91) Lesions Shape Round 54 (59%) Oval 32 (35%) Lobular 5 (6%) Margin Smooth 84 (92%) Irregular 7 (8%) Signal intensity (T1W images) Hypointense 10 (11%) Isointense 79 (87%) Mixed isointense to hyperintense 2 (2%) Signal intensity (T2W images) Isointense 76 (84%) Hyperintense 15 (16%) Internal mass enhancement Homogenous 73 (80%) Inhomogenous 7 (8%) Rim enhancement 11 (12%) Time–signal intensity curve pattern Type 1 11 (18%) Type 2 32 (52%) Type 3 19 (30%)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Diagnosis and Follow-Up
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 7
Comparison of Reported Studies Regarding Radiologic Findings of Breast Metastases
Study Patients Radiologic Investigations (Patients) CT Mammography Ultrasound Doppler MRI Bohman et al ∗ 11 — 11 — — — McCrea et al ∗ 8 — 8 — — — Derchi et al 6 — — 6 — — Hebert et al 21 — 5 — — — Vergier et al 8 — 8 8 — — Muttarak et al 7 — 7 6 — — Yeh et al 15 — — 11 — — Noguera et al ∗ 22 4 18 11 — 1 Present study 51 38 37 43 20 24
CT, computed tomography; MRI, magnetic resonance imaging.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
References
1. Bartella L., Kaye J., Perry N.M., et. al.: Metastases to the breast revisited: radiological-histopathological correlation. Clin Radiol 2003; 58: pp. 524-531.
2. Yeh C.N., Lin C.H., Chen M.F.: Clinical and ultrasonographic characteristics of breast metastases from extramammary malignancies. Am Surg 2004; 70: pp. 287-290.
3. Hajdu S.I., Urban J.A.: Cancers metastatic to the breast. Cancer 1972; 29: pp. 1691-1696.
4. Yang W.T., Muttarak M., Ho L.W.: Nonmammary malignancies of the breast: ultrasound, CT, and MRI. Semin Ultrasound CT MRI 2000; 21: pp. 375-394.
5. Vergier B., Trojani M., de Mascarel I., et. al.: Metastases to the breast: differential diagnosis from primary breast carcinoma. J Surg Oncol 1991; 48: pp. 112-116.
6. Harvey J.A.: Unusual breast cancers: useful clues to expanding the differential diagnosis. Radiology 2007; 242: pp. 683-694.
7. Alva S., Shetty-Alva N.: An update of tumor metastasis to the breast. Arch Surg 1999; 134: pp. 450.
8. Wood B., Sterrett G., Frost F., et. al.: Diagnosis of extramammary malignancy metastatic to the breast by fine needle biopsy. Pathology 2008; 40: pp. 345-351.
9. Abrams H.L., Spiro R., Goldstein N.: Metastases in carcinoma. Analysis of 1000 autopsied cases. Cancer 1950; 3: pp. 74-85.
10. Hejmadi R.K., Day L.J., Young J.A.: Extramammary metastatic neoplasms in the breast: a cytomorphological study of 11 cases. Cytopathology 2003; 14: pp. 191-194.
11. Silverman J.F., Feldman P.S., Covell J.L., et. al.: Fine needle aspiration cytology of neoplasms metastatic to the breast. Acta Cytol 1987; 31: pp. 291-300.
12. Georgiannos S.N., Chin J., Goode A.W., et. al.: Secondary neoplasms of the breast: a survey of the 20th Century. Cancer 2001; 92: pp. 2259-2266.
13. Amichetti M., Perani B., Boi S.: Metastases to the breast from extramammary malignancies. Oncology 1990; 47: pp. 257-260.
14. Vaughan A., Dietz J.R., Moley J.F., et. al.: Metastatic disease to the breast: the Washington University experience. World J Surg Oncol 2007; 5: pp. 74.
15. Smyrniotis V., Theodosopoulos T., Marinis A., et. al.: Metastatic disease in the breast from nonmammary neoplasms. Eur J Gynaecol Oncol 2005; 26: pp. 547-550.
16. Williams S.A., Ehlers R.A., Hunt K.K., et. al.: Metastases to the breast from nonbreast solid neoplasms: presentation and determinants of survival. Cancer 2007; 110: pp. 731-737.
17. David O., Gattuso P., Razan W., et. al.: Unusual cases of metastases to the breast. A report of 17 cases diagnosed by fine needle aspiration. Acta Cytol 2002; 46: pp. 377-385.
18. Bohman L.G., Bassett L.W., Gold R.H., et. al.: Breast metastases from extramammary malignancies. Radiology 1982; 144: pp. 309-312.
19. McCrea E.S., Johnston C., Haney P.J.: Metastases to the breast. AJR Am J Roentgenol 1983; 141: pp. 685-690.
20. Derchi L.E., Rizzatto G., Giuseppetti G.M., et. al.: Metastatic tumors in the breast: sonographic findings. J Ultrasound Med 1985; 4: pp. 69-74.
21. Hebert G., Ouimet-Oliva D., Paquin F., et. al.: Diffuse metastatic involvement of the breast. Can Assoc Radiol J 1991; 42: pp. 353-356.
22. Toombs B.D., Kalisher L.: Metastatic disease to the breast: clinical, pathologic, and radiographic features. AJR Am J Roentgenol 1977; 129: pp. 673-676.
23. Wurdinger S., Schütz K., Fuchs D., et. al.: Two cases of metastases to the breast on MR mammography. Eur Radiol 2001; 11: pp. 802-806.
24. Lehman C., Holt S., Peacock S., et. al.: Use of the American College of Radiology BI-RADS guidelines by community radiologists: concordance of assessments and recommendations assigned to screening mammograms. AJR Am J Roentgenol 2002; 179: pp. 15-20.
25. Kim E.K., Ko K.H., Oh K.K., et. al.: Clinical application of the BI-RADS final assessment to breast sonography in conjunction with mammography. AJR Am J Roentgenol 2008; 190: pp. 1209-1215.
26. Kuhl C.K., Mielcareck P., Leutner C., et. al.: Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions. Radiology 1999; 211: pp. 101-110.
27. Szabo B.K., Aspelin P., Wiberg M.K., et. al.: Dynamic MR imaging of the breast: analysis of kinetic and morphologic diagnostic criteria. Acta Radiol 2003; 44: pp. 379-386.
28. Baum F., Fischer U., Vosshenrich R., et. al.: Classification of hypervascularized lesions in CE MR imaging of the breast. Eur Radiol 2002; 12: pp. 1087-1092.
29. Macura K.J., Ouwerkerk R., Jacobs M.A., et. al.: Patterns of enhancement on breast MR images: interpretation and imaging pitfalls. Radiographics 2006; 26: pp. 1719-1734.
30. Erguvan-Dogan B., Whitman G.J., Kushwaha A.C., et. al.: BI-RADS-MRI: a primer. AJR Am J Roentgenol 2006; 187: pp. W152-W160.
31. Morris E.A.: Breast MR imaging lexicon updated. Magn Res Imaging Clin N Am 2006; 14: pp. 293-303.
32. Sneige N., Zachariah S., Fanning T., et. al.: Fine-needle aspiration cytology of metastatic neoplasms in the breast. Am J Clin Pathol 1989; 92: pp. 27-35.
33. Van Hoeven K.H., Hibbard C.A., Flax H., et. al.: Metastatic malignant neoplasms and secondary lymphomatous involvement of the breast: a study of 43 cases. Pathol Annu 1993; 28: pp. 221-241.
34. Lee S.K., Kim W.W., Kim S.H., et. al.: Characteristics of metastasis in the breast from extramammary malignancies. J Surg Oncol 2010; 101: pp. 137-140.
35. Noguera J.J., Martinez-Miravete P., Idoate F., et. al.: Metastases to the breast: a review of 33 cases. Australas Radiol 2007; 51: pp. 133-138.
36. Muttarak M., Nimmonrat A., Chaiwun B.: Metastatic carcinoma to the male and female breast. Australas Radiol 1998; 42: pp. 16-19.
37. Vizcaino I., Torregrosa A., Higueras V., et. al.: Metastasis to the breast from extramammary malignancies: a report of four cases and a review of literature. Eur Radiol 2001; 11: pp. 1659-1665.
38. Soo M.S., Williford M.E., Elenberger C.D.: Medullary thyroid carcinoma metastatic to the breast: mammographic appearance. AJR Am J Roentgenol 1995; 165: pp. 65-66.
39. Chateil J.F., Arboucalot F., Pérel Y., et. al.: Breast metastases in adolescent girls: US findings. Pediatr Radiol 1998; 28: pp. 832-835.
40. Lee W.K., Cawson J.N., Hill P.A., et. al.: Renal cell carcinoma metastasis to the breast: mammographic, sonographic, CT, and pathologic correlation. Breast J 2007; 13: pp. 316-317.
41. Kim S.M., Ko E.Y., Han B.K., et. al.: Metastatic thymoma of the breast. Korean J Radiol 2008; 9: pp. 80-83.
42. Francois C., Rangachari B., Bova D.: Mammography and sonography of pathologically proven adrenal cortical carcinoma metastatic to the breast. AJR Am J Roentgenol 2005; 184: pp. 1279-1281.
43. Ashkar L., Mesurolle I., Trembley F.: Sonographic target sign and rim enhancement on magnetic resonance imaging in metastatic melanoma to the breast. Australas Radiol 2006; 50: pp. 237-240.
44. Yang W.T., Kwan W.H., Chow L.T., et. al.: Unusual sonographic appearance with color Doppler imaging of bilateral breast metastases in a patient with alveolar rhabdomyosarcoma of an extremity. J Ultrasound Med 1996; 15: pp. 531-533.
45. Tukel S., Dogan B.E., Ozcan H.: Alveolar rhabdomyosarcoma metastatic to the breast. Curr Probl Diagn Radiol 2003; 32: pp. 102-104.
46. Kalra N., Ojili V., Gulati M., et. al.: Metastatic choriocarcinoma to the breast: appearance on mammography and Doppler sonography. AJR Am J Roentgenol 2005; 184: pp. S53-S55.
47. Upalakalin J.N., Collins L.C., Tawa N., et. al.: Carcinoid tumors in the breast. Am J Surg 2006; 191: pp. 799-805.
48. Jakovljevic B., Stevanovic O., Bacic G.: Metastases to the breast from small-cell lung cancer: MR findings. A case report. Acta Radiol 2003; 44: pp. 485-488.
49. Perlet C., Sittek H., Forstpointner R., et. al.: Metastases to the breast from rhabdomyosarcoma: appearances on MRI. Eur Radiol 1999; 9: pp. 1113-1116.
50. Kelkar P.S., Helbich T.H., Becherer A., et. al.: Solitary breast metastasis as the first sign of a squamous cell carcinoma of the cervix: imaging findings. Eur J Radiol 1997; 24: pp. 159-162.
51. Briest S., Horn L.C., Haupt R., et. al.: Metastasizing signet ring cell carcinoma of the stomach-mimicking bilateral inflammatory breast cancer. Gynecol Oncol 1999; 74: pp. 491-494.
52. Birjawi G.A., Haddad M.C., Tawil A.N., et. al.: Metastatic rhabdomyosarcoma to the breast. Eur Radiol 2001; 11: pp. 555-558.
53. Ho L.W., Wong K.P., Chan J.H., et. al.: MR appearance of metastatic melanotic melanoma in the breast. Clin Radiol 2000; 55: pp. 572-573.
54. Özgüroglu M., Ersavasti G., Ilvan S., et. al.: Bilateral inflammatory breast metastases of epithelial ovarian cancer. Am J Clin Oncol 1999; 22: pp. 408-410.
55. Gomori J.M., Grossman R.I., Shields J.A., et. al.: Choroidal melanomas: correlation of NMR spectroscopy and MR imaging. Radiology 1986; 158: pp. 443-445.
56. Woodruff W.W., Djang W.T., McLendon R.E., et. al.: Intracerebral malignant melanoma: high-field-strength MR imaging. Radiology 1987; 165: pp. 209-213.