Objectives
We proposed a modification of the ACR Lung Imaging Reporting and Data System (Lung-RADS) to clarify the characteristics of subsolid nodules with categories 1–11, and to compare the diagnostic accuracy with Lung-RADS and National Lung Screening Trial criteria in an Asian population with high prevalence of adenocarcinoma.
Methods
We analyzed a retrospective cohort of 1978 consecutive healthy subjects (72.8% nonsmoker) who underwent low-dose computed tomography from August 2013 to October 2014 (1084 men, 894 women). Lung-RADS categories 2 and 3 were modified to include subcategories of 2A/2B/2C and 3A/3B/3C, respectively. Clinical information and nodule characteristics were recorded. Receiver operating characteristic curves were used to compare diagnostic accuracy at different cutoffs.
Results
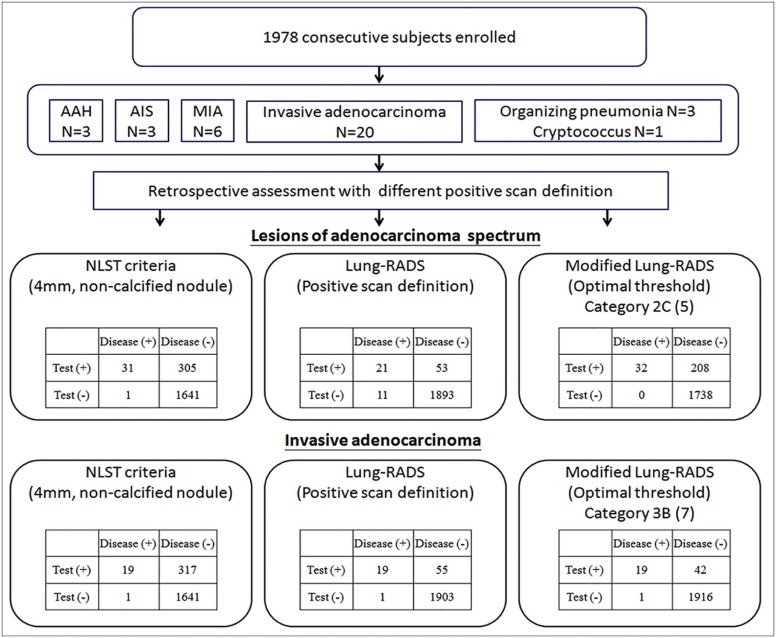
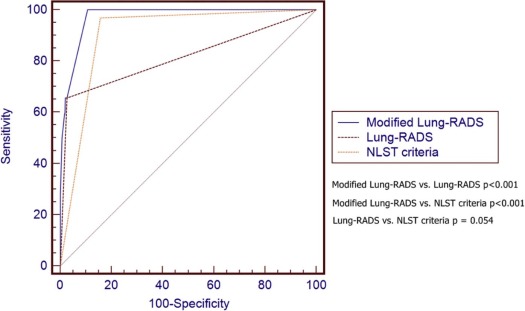
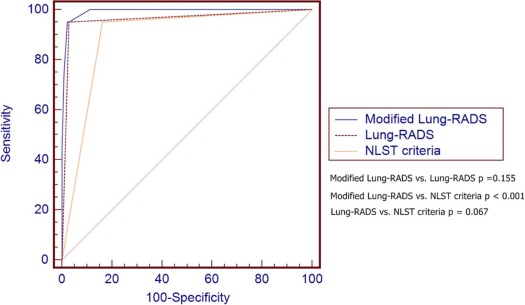
Thirty-two subjects (30 nonsmokers) had pathology-proven adenocarcinoma spectrum lesions in the follow-up period (1.6 ± 0.5 years). Modified Lung-RADS, using modified Lung-RADS category 2C as cutoff, had an area under the curve (AUC) of 0.973 in predicting adenocarcinoma spectrum lesions (sensitivity of 100%, specificity of 89.3%), which was significantly higher than that of Lung-RADS (AUC = 0.815, P < .001) and National Lung Screening Trial (AUC = 0.906, P < .001). Furthermore, modified Lung-RADS showed an AUC of 0.992 in predicting invasive adenocarcinoma (sensitivity of 95%, specificity of 97.8%) when category 3B was used as cutoff.
Conclusions
Modified Lung-RADS may substantially improve sensitivity while maintaining specificity for detection of adenocarcinoma spectrum lesions in an Asian population. Compared to Lung-RADS, it has enhanced ability to differentiate invasive from indolent adenocarcinoma by more refined subclassification of subsolid nodules using two cutoff values of category 2C and 3B. The effect of using modified Lung-RADS in clinical practice must be carefully studied in prospective large cohort studies.
Introduction
The National Lung Screening Trial (NLST) demonstrated that annual lung screening of high-risk population with low-dose computed tomography (LDCT) results in a 20% reduction in lung cancer-specific mortality compared to screening with chest radiography . However, a major concern is the high false-positive rate of NLST by setting the cutoff threshold of positive scan at 4 mm for noncalcified lung nodule . In an effort to reduce false-positive rate and standardize reporting of lung cancer screening examinations, the American College of Radiology (ACR) in 2014 released the Lung Imaging Reporting and Data System (Lung-RADS) . Recent studies have demonstrated that Lung-RADS, compared to NLST criteria, substantially reduces the false-positive result rate with a small corresponding decrease in sensitivity .
In general, cancer cases that are falsely classified as negative by Lung-RADS and classified as positive screen based on NLST criteria are speculated to represent less aggressive lesions, such as minimally invasive adenocarcinoma (MIA) or adenocarcinoma in situ (AIS) . However, Lung-RADS was designed to be used in the United States where screening programs target high-risk smokers, and the diagnostic performance of Lung-RADS in populations with high prevalence of nonsmoking associated lung cancer, such as in China, Taiwan, Korea, and Japan, is unclear . Lung cancer is the leading cause of death among all cancer types in Taiwan, accounting for 19.7% of cancer mortality in 2012. In Taiwan, more than 95% of women with lung cancer are nonsmokers, and the majority of these patients have adenocarcinomas (about 83%) . More than half of women with lung cancer are diagnosed at advanced stages.
Get Radiology Tree app to read full this article<
Materials and Methods
Study Cohort
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
LDCT Image Acquisition and Interpretation
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Retrospective Reclassification of Scans According to the Definition of NLST, Lung-RADS, and Modified Lung-RADS Criteria
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
TABLE 1
Summary of Lung-RADS Classification and Modified Lung-RADS Classification
Lung-RADS Baseline Screening Category Modified Lung-RADS Category Modified Lung-RADS Categories in Numbers 1 No nodules 1A (no nodules) 1 Nodules with calcification 1B (nodules with calcification) 2 2 Solid: <6 mm 2A (Solid: <6 mm) 3 Part solid: <6 mm 2B (Part solid: <6 mm) 4 GGN: <20 mm 2C (GGN: <20 mm) 5 3 Solid: ≥6 to <8 mm 3A (Solid: ≥6 to <8 mm) 6 Part solid: ≥6 mm with solid component <6 mm 3B (Part solid: ≥6 mm with solid component <6 mm) 7 GGN: ≥20 mm 3C (GGN: ≥20 mm) 8 4A Solid: ≥8 mm to <15 mm 4A(Correspond to Lung-RADS 4A) 9 Part solid: ≥8 mm with solid component ≥6 and <8 mm 4B Solid: ≥15 mm 4B(Correspond to Lung-RADS 4B) 10 Part solid: Solid component ≥8 mm 4X Category 3 or 4 nodules with additional features; imaging findings that increase suspicion of malignancy 4X (Correspond to Lung-RADS 4X) 11
ACR, American College of Radiology; GGN, ground-glass nodule; Lung-RADS, the ACR Lung Imaging Reporting and Data System; modified Lung-RADS, a modified 11-category scheme classification system from the Lung-RADS.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Demographics and Clinical Characteristics
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
TABLE 2
Clinical Characteristics of the Total Screened Population at the Baseline
Characteristics Mean, Median, Range/n (%) Ethnic group Asian (%) 100% Gender Male, n (%) 1084 (54.80%) Female, n (%) 894 (45.20%) Age (y) 56.56, 57, 40–80 BMI (kg/m 2 ) 24.33 ± 3.54 (13–42) Family history of lung cancer 20.40% Family history of other cancer 31.40% COPD 3.40% TB 2.80% NLST criteria 149 (7.50%) Smoking status Nonsmoker 1440 (72.80%) Smoker 211 (10.70%) Quit 327 (16.50%) Pack-years of smoking Smoker 23.18 ± 19.95, 20, 1–120 Quit 6.60 ± 13.12, 6.6, 0–120 Lung-RADS 1 1559 (78.81%) 2 345 (17.44%) 3 21 (1.06%) 4 53 (2.60%) Lung-RADS Negative (1–2) 1904 (96.30%) Positive (3–4) 74 (3.70%) Lung adenocarcinoma spectrum type AAH 3 (9.37%) AIS 3 (9.37%) MIA 6 (18.75%) Invasive adenocarcinoma 20 (62.50%) Lung cancer stage Stage 0 6 (18.75%) Stage I 23 (71.87%) Stage II 2 (6.25%) Stage III 0 (0%) Stage IV 1 (3.10%) Eligible risk factor for 32 subjects with lung adenocarcinoma spectrum Eligible for NLST criteria 1 (3.10%) Smoking-related (not eligible for NLST criteria) 1 (3.10%) Nonsmoking related 30 (93.80%)
AAH, atypical adenomatous hyperplasia; ACR, American College of Radiology; AIS, adenocarcinoma in situ; BMI, body mass index; COPD, chronic obstructive pulmonary disease; Lung-RADS, The ACR Lung Imaging Reporting and Data System; MIA, minimally invasive adenocarcinoma; NLST, National Lung Screening Trial; TB, tuberculosis.
Data are presented as mean ± SD, median, or range/n (%).
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
TABLE 3
Comparison Between Lung-RADS Classification and Modified Lung-RADS Classification in Baseline Screening of 1978 Subjects Enrolled in This Study
Lung-RADS Modified Lung-RADS Cancer Diagnosed, n (%) All Classifications, n (%) Percentage of ADE and MIA Stage Distribution 1 1A (1) 0 (0%) 1301 (65.70%) 0 N/A 1B (2) 0 (0%) 258 (13.04%) 0 N/A 2 2A (3) 0 (0%) 173 (8.76%) 0 N/A 2B (4) 0 (0%) 6 (0.30%) 0 N/A 2C (5) 11 (6.60%) 166 (8.39%) 45.40% (5/11) Stage I (5); stage 0 (6) 3 3A (6) 0 (0%) 13 (0.65%) 0 N/A 3B (7) 2 (28.50%) 7 (0.35%) 100% (2/2) Stage I (2) 3C (8) 0 (0%) 1 (0.05%) 0 N/A 4A 4A (9) 3 (13.60%) 22 (1.10%) 100% (3/3) Stage I (3) 4B 4B (10) 7 (35.00%) 20 (1.01%) 100% (7/7) Stage I (7) 4X 4X (11) 9 (81.80%) 11 (0.55%) 100% (9/9) Stage I (6); stage II (2); stage IV (1)
ACR, American College of Radiology; ADE, invasive adenocarcinoma; Lung-RADS, The ACR Lung Imaging Reporting and Data System; MIA, minimally invasive adenocarcinoma; N/A, not applicable.
Cancer diagnosed: lung adenocarcinoma spectrum lesions.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Predictive Ability for Lung Adenocarcinoma Spectrum Lesions
Get Radiology Tree app to read full this article<
TABLE 4
Comparison of Diagnostic Accuracy for Lung Adenocarcinoma Spectrum Lesions at Different Size Criteria Definition and Cutoff Values
Positive Scan Definition Cutoff Value AUC Sensitivity (95% CI) Specificity (95% CI) DA (%) PPV (95% CI) NPV (95% CI) LR+ LR− Lung-RADS Positive scan results † 0.815 (0.797, 0.831) ‡ 65.6 (46.8, 81.4) 97.2 (96.5, 98.0) 96.76 28.4 (18.5, 40.1) 99.4 (99.0, 99.7) 24.100 0.350 Modified Lung-RADS Category 2C \* 0.973 (0.965, 0.980) ‡ 100.0 (89.1, 100.0) 89.3 (87.9, 90.6) 89.48 13.3 (9.3, 18.3) 100.0 (99.8, 100.0) 9.360 0 Size criteria 4 mm 4 mm 0.875 (0.860, 0.890) ‡ 100.0 (89.1, 100.0) 75.0 (73.1, 77.0) 75.36 6.2 (4.3, 8.6) 100.0 (99.7, 100.0) 4.010 0 Size criteria 6 mm 6 mm 0.919 (0.907, 0.931) ‡ 93.7 (79.2, 99.2) 90.1 (88.7, 91.4) 90.15 13.5 (9.3, 18.7) 99.9 (99.6, 100.0) 9.500 0.069 Size criteria 8 mm 8 mm 0.914 (0.900, 0.926) ‡ 87.50 (71.0, 96.5) 95.2 (94.2, 96.1) 95.07 23.1 (16.0, 31.7) 99.8 (99.4, 99.9) 18.310 0.130 NLST criteria 4 mm, noncalcified 0.906 (0.892, 0.919) ‡ 96.8 (83.8, 99.9) 84.3 (82.6, 85.9) 84.52 9.2 (6.4, 12.8) 99.9 (99.7, 100.0) 6.180 0.037
ACR, American College of Radiology; AUC, area under the curve; AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; CI, confidence interval; DA, diagnostic accuracy; LR+, positive likelihood ratio; LR−, negative likelihood ratio; Lung-RADS, the ACR Lung Imaging Reporting and Data System; MIA, minimal invasive adenocarcinoma; NLST, National Lung Screening Trial; NPV, negative predictive value; PPV, positive predictive value.
Lung adenocarcinoma spectrum classification, including AAH, AIS, MIA, and invasive adenocarcinoma.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Predictive Ability for Invasive Lung Adenocarcinoma
Get Radiology Tree app to read full this article<
TABLE 5
Comparison of Diagnostic Accuracy for Invasive Adenocarcinoma at Different Optimal Cutoff Values Comparing the Predictive Ability of NLST, Lung-RADS, and Modified Lung-RADS Criteria
Positive Scan Definition Cutoff Value AUC Sensitivity (95% CI) Specificity (95% CI) DA (%) PPV (95% CI) NPV (95% CI) LR+ LR− NLST criteria 4 mm, Noncalcified 0.973 (0.965, 0.980) ‡ 95.0 (75.1, 99.9) 83.8 (82.1, 85.4) 83.92 5.7 (3.4, 8.7) 99.9 (99.7, 100.0) 5.870 0.060 Modified Lung-RADS Category 3B \* 0.992 (0.987, 0.995) ‡ 95.0 (75.1, 99.9) 97.8 (97.1, 98.4) 97.82 31.1 (19.9, 44.3) 99.9 (99.7, 100.0) 33.820 0.051 Lung-RADS Positive scan † 0.961 (0.951, 0.969) ‡ 95.0 (75.1, 99.9) 97.1 (96.4, 97.9) 97.16 25.7 (16.2, 37.2) 99.9 (99.7, 100.0) 33.820 0.051
ACR, American College of Radiology; AUC, area under the curve; CI, confidence interval; DA, diagnostic accuracy; LR+, positive likelihood ratio; LR−, negative likelihood ratio; Lung-RADS, the ACR Lung Imaging Reporting and Data System; modified Lung-RADS, a modified 11-category scheme classification system from the Lung-RADS proposed in this study; NLST, National Lung Screening Trial; NPV, negative predictive value; PPV, positive predictive value.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Supplementary Data
Get Radiology Tree app to read full this article<
Table S1
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Team TNLSTR : Reduced lung-cancer mortality with low-dose computed tomographic screening. NEJM 2011; 365: pp. 395-409.
2. Pinsky P.F., Gierada D.S., Nath H., et. al.: ROC curves for low-dose CT in the National Lung Screening Trial. J Med Screen 2013; 20: pp. 165-168.
3. Gierada D.S., Pinsky P., Nath H., et. al.: Projected outcomes using different nodule sizes to define a positive CT lung cancer screening examination. J Natl Cancer Inst 2014; 106: pii: dju284
4. American College of Radiology : Lung CT screening reporting and data system (Lung-RADS). Available at: www.acr.org/Quality-Safety/Resources/LungRADS
5. Pinsky P.F., Gierada D.S., Black W., et. al.: Performance of lung-RADS in the National Lung Screening Trial: a retrospective assessment performance of lung-RADS in the NLST. Ann Intern Med 2015; 162: pp. 485-491.
6. Wu F.-Z., Huang Y.-L., Wu C.C., et. al.: Assessment of selection criteria for low-dose lung screening CT among Asian Ethnic Groups in Taiwan: from mass screening to specific risk-based screening for non-smoker lung cancer. Clin Lung Cancer 2016; 17: pp. e45-e56.
7. Detterbeck F.C., Marom E.M., Arenberg D.A., et. al.: The IASLC lung cancer staging project: background data and proposals for the application of TNM staging rules to lung cancer presenting as multiple nodules with ground glass or lepidic features or a pneumonic type of involvement in the forthcoming eighth edition of the TNM classification. J Thorac Oncol 2016; 11: pp. 666-680.
8. 2015 Health Promotion Administration, Ministry of Health and Welfare : Taiwan Cancer Registry, Cancer Registry annual report 2012.2015.Health Promotion AdministrationTaiwan
9. Chen K.-Y., Chang C.-H., Yu C.-J., et. al.: Distribution according to histologic type and outcome by gender and age group in Taiwanese patients with lung carcinoma. Cancer 2005; 103: pp. 2566-2574.
10. Ha S.Y., Choi S.-J., Cho J.H., et. al.: Lung cancer in never-smoker Asian females is driven by oncogenic mutations, most often involving EGFR. Oncotarget 2015; 6: pp. 5465-5474.
11. Lin K.-F., Wu H.-F., Huang W.-C., et. al.: Propensity score analysis of lung cancer risk in a population with high prevalence of non-smoking related lung cancer. BMC Pulm Med 2017; 17: pp. 120.
12. Nawa T., Nakagawa T., Mizoue T., et. al.: A decrease in lung cancer mortality following the introduction of low-dose chest CT screening in Hitachi, Japan. Lung Cancer 2012; 78: pp. 225-228.
13. Kazerooni E.A., Austin J.H., Black W.C., et. al.: ACR-STR practice parameter for the performance and reporting of lung cancer screening thoracic computed tomography (CT): 2014 (Resolution 4). J Thorac Imaging 2014; 29: pp. 310-316.
14. MacMahon H., Austin J.H.M., Gamsu G., et. al.: Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner society. Radiology 2005; 237: pp. 395-400.
15. Naidich D.P., Bankier A.A., MacMahon H., et. al.: Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner society. Radiology 2013; 266: pp. 304-317.
16. MacMahon H., Naidich D.P., Goo J.M., et. al.: Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner society. Radiology 2017; 2017: pp. 161659.
17. DeLong E.R., DeLong D.M., Clarke-Pearson D.L.: Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: pp. 837-845.
18. Travis W.D., Brambilla E., Noguchi M., et. al.: International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol 2011; 6: pp. 244-285.
19. McKee B.J., Regis S.M., McKee A.B., et. al.: Performance of ACR Lung-RADS in a clinical CT lung screening program. J Am Coll Radiol 2015; 12: pp. 273-276.
20. Yip R., Yankelevitz D.F., Hu M., et. al.: Lung cancer deaths in the National Lung Screening Trial attributed to nonsolid nodules. Radiology 2016; 281: pp. 589-596.
21. Kakinuma R., Noguchi M., Ashizawa K., et. al.: Natural history of pulmonary subsolid nodules: a prospective multicenter study. J Thorac Oncol 2016; 11: pp. 1012-1028.
22. Henschke C.I., Yankelevitz D.F., Mirtcheva R., et. al.: CT screening for lung cancer. Am J Roentgenol 2002; 178: pp. 1053-1057.
23. Nakata M., Saeki H., Takata I., et. al.: Focal ground-glass opacity detected by low-dose helical CT. Chest 2002; 121: pp. 1464-1467.
24. Cho J., Kim E.S., Kim S.J., et. al.: Long-term follow-up of small pulmonary ground-glass nodules stable for 3 years: implications of the proper follow-up period and risk factors for subsequent growth. J Thorac Oncol 2016; 11: pp. 1453-1459.
25. Chang B., Hwang J.H., Choi Y.H., et. al.: Natural history of pure ground-glass opacity lung nodules detected by low-dose CT scan. Chest 2013; 143: pp. 172-178.
26. Matsuguma H., Mori K., Nakahara R., et. al.: Characteristics of subsolid pulmonary nodules showing growth during follow-up with CT scanning. Chest 2013; 143: pp. 436-443.
27. Lee S.M., Park C.M., Goo J.M., et. al.: Invasive pulmonary adenocarcinomas versus preinvasive lesions appearing as ground-glass nodules: differentiation by using CT features. Radiology 2013; 268: pp. 265-273.
28. Wu F.-Z., Chen P.-A., Wu C.C., et. al.: Semiquantative visual assessment of sub-solid pulmonary nodules ≦3 cm in differentiation of lung adenocarcinoma spectrum. Sci Rep 2017; 7: pp. 15790.
29. Liu L.H., Liu M., Wei R., et. al.: CT findings of persistent pure ground glass opacity: can we predict the invasiveness?. Asian Pac J Cancer Prev 2015; 16: pp. 1925-1928.
30. Jin X., Zhao S.-H., Gao J., et. al.: CT characteristics and pathological implications of early stage (T1N0M0) lung adenocarcinoma with pure ground-glass opacity. Eur Radiol 2015; 25: pp. 2532-2540.
31. Lee H.Y., Choi Y.-L., Lee K.S., et. al.: Pure ground-glass opacity neoplastic lung nodules: histopathology, imaging, and management. Am J Roentgenol 2014; 202: pp. W224-W233.
32. Lim H.J., Ahn S., Lee K.S., et. al.: Persistent pure ground-glass opacity lung nodules ≥ 10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest 2013; 144: pp. 1291-1299.
33. Fang W., Xiang Y., Zhong C., et. al.: The IASLC/ATS/ERS classification of lung adenocarcinoma—a surgical point of view. J Thorac Dis 2014; 6: pp. S552-S560.
34. Scholten E.T., de Jong P.A., de Hoop B., et. al.: Towards a close computed tomography monitoring approach for screen detected subsolid pulmonary nodules?. Eur Respir J 2015; 45: pp. 765-773.
35. Lee J.H., Park C.M., Kim H., et. al.: Persistent part-solid nodules with solid part of 5 mm or smaller: can the “follow-up and surgical resection after interval growth” policy have a negative effect on patient prognosis?. Eur Radiol 2016; 27: pp. 195-202.
36. Sverzellati N., Silva M., Calareso G., et. al.: Low-dose computed tomography for lung cancer screening: comparison of performance between annual and biennial screen. Eur Radiol 2016; 26: pp. 3821-3829.
37. Sox H.C.: Implementing lung cancer screening under Medicare: the last chance to get it right?. JAMA 2014; 312: pp. 1206-1207.
38. Volk R.J., Hawk E., Bevers T.B.: Should CMS cover lung cancer screening for the fully informed patient?. JAMA 2014; 312: pp. 1193-1194.
39. van Klaveren R.J., Oudkerk M., Prokop M., et. al.: Management of lung nodules detected by volume CT scanning. NEJM 2009; 361: pp. 2221-2229.
40. Horeweg N., Scholten E.T., de Jong P.A., et. al.: Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol 2014; 15: pp. 1342-1350.
41. Detterbeck F.C., Mazzone P.J., Naidich D.P., et. al.: Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013; 143: pp. e78S-e92S.
42. Taipei TBoHP. Bureau of Health Promotion. Report from Adult Smoking Behavior Surveillance System.
43. Yi C.A., Lee K.S., Shin M.-H., et. al.: Low-dose CT screening in an Asian population with diverse risk for lung cancer: a retrospective cohort study. Eur Radiol 2015; 25: pp. 2335-2345.
44. Chen C.-Y., Chen C.-H., Shen T.-C., et. al.: Lung cancer screening with low-dose computed tomography: experiences from a tertiary hospital in Taiwan. J Formos Med Assoc 2016; 115: pp. 163-170.
45. Green D.B., Pua B.B., Crawford C.B., et. al.: Screening for lung cancer: communicating with patients. Am J Roentgenol 2017; pp. 1-6.
46. Fenton J.J., Deyo R.A.: Patient self-referral for radiologic screening tests: clinical and ethical concerns. J Am Board Fam Pract 2003; 16: pp. 494-501.