Purpose
Systemic lupus erythematosus (SLE) is a predominantly female autoimmune disease that can affect the central nervous system. Neuropsychiatric symptoms are found in 25–70% of SLE patients. Using diffusion tensor imaging, various studies have reported changes in white matter integrity in SLE patients with neuropsychiatric symptoms (NPSLE patients). The purpose of this study was to investigate if changes can be detected in the individual white matter tracts in SLE patients regardless if neuropsychiatric symptoms are present or not.
Materials and Methods
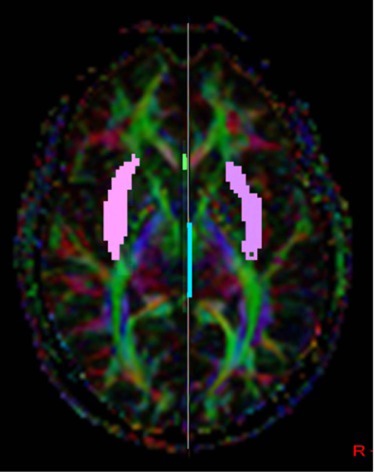
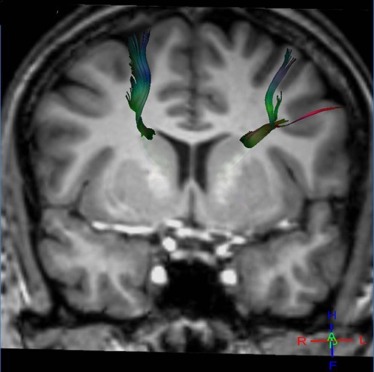
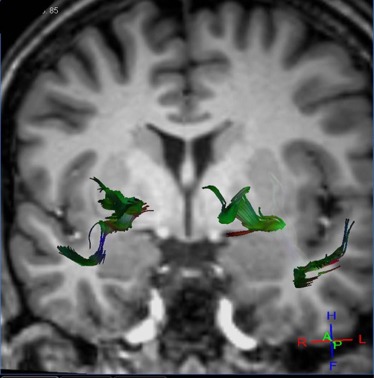
Magnetic resonance diffusion tractography in several individual white matter tracts that are involved in language and memory tasks, including tracts to cortical association areas, was applied in 21 patients with NPSLE (mean age: 40.7 ± 12.8 years; range: 22–67 years), 18 patients with non-neurologic systemic lupus erythematosus (non-NPSLE) (mean age: 40.6 ± 12 years; range: 22–67 years), and 20 healthy control (HC) individuals (mean age: 40.64 ± 12.7 years; range: 19–60 years). Additional patients were evaluated; however, because of the inability to complete the scans required, they were excluded from the study. The fractional anisotropy of individual fiber tracts was measured and correlated with cognitive function and lupus disease severity index (Systemic Lupus Erythematosus Disease Activity Index [SLEDAI]) to assess predictability and diagnostic value of these measures for NPSLE.
Results
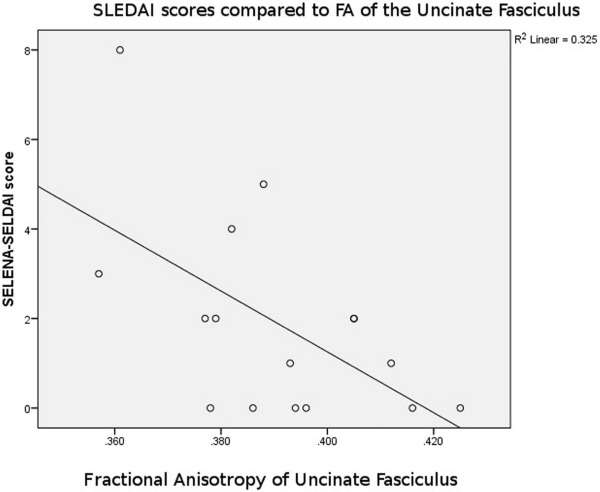
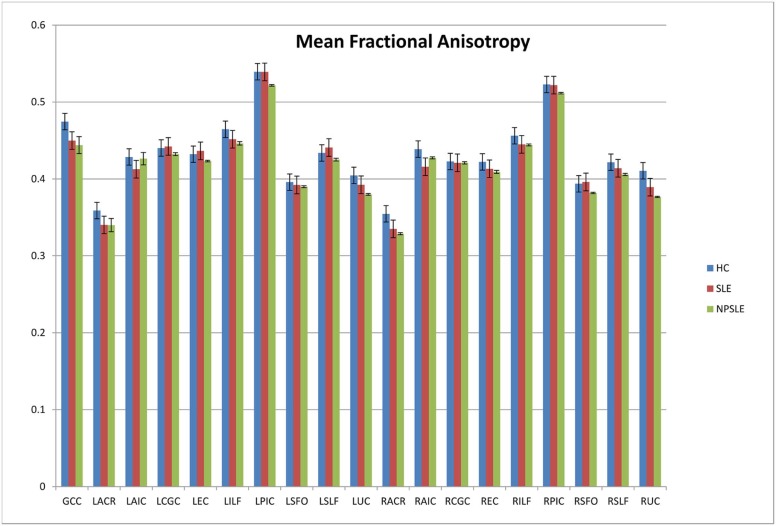
Analyses of variance of the tractography data from the analysis of 21 tracts revealed decreased fractional anisotropy in uncinate fasciculus in the NPSLE patients when compared to non-NPSLE lupus patients and HC individuals ( P = 0.002). Non-NPSLE patients also demonstrated decreased fractional anisotropy when compared to healthy patients ( P = 0.03). Decreased fractional anisotropy was also identified in the corpus callosum and corona radiata in NPSLE patients when compared to HC individuals; however, these tracts did not show a significant difference between NPSLE and non-NPSLE patients. Decreased fractional anisotropy in the uncinate fasciculus correlated with low SLEDAI score (R 2 = 0.32).
Conclusions
Diffusion tensor tractography corroborates findings of decreased white matter integrity within the anterior corona radiate as well as the corpus callosum as previously described. Specifically, our study identified changes in the uncinate fasciculus in NPSLE and non-NPSLE patients that correlate with clinical changes (SLEDAI scores) and are independent of conventional T2 lesion burden.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease afflicting multiple organs of the body and is affecting 0.05–0.1% of the general population . Neuropsychiatric systemic lupus erythematosus (NPSLE) has been classified as a complex neurologic disorder categorized by 19 diagnostic criteria set forth by the American College of Rheumatology (ACR) based on the presence of SLE and neuropsychiatric conditions. NPSLE is reported to occur in 30–72% of patients with SLE and is associated with increased morbidity and mortality .
Magnetic resonance imaging (MRI) has become routine in the clinical management of SLE patients with or withoutneuropsychiatric conditions to evaluate for complications of the disease that may contribute to neurologic sequela. MRI is also helpful in diagnosing or excluding cerebral pathologies, such as hemorrhage, cerebral venous thrombosis, or stroke . Conventional MR sequences such as T2-weighted and fluid attenuation inversion recovery (FLAIR) have demonstrated focal hyperintense lesions in periventricular white matter in up to 70% of NPSLE patients ; however, these hyperintense lesions are non-specific and can be present in both NPSLE and non-NPSLE patients, as well as in other unrelated conditions such as small vessel disease, aging, or demyelinating disease . White matter T2 signal changes are also a relatively common finding in cognitively healthy elderly .
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and Methods
Patients and Healthy Control Subjects
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Data Acquisition
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Imaging Post-processing and Analysis
DTI Post-processing
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
The Tract-based White Matter Burden Evaluation
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Morphology and Atrophy
Get Radiology Tree app to read full this article<
Tract-based White Matter Burden
Get Radiology Tree app to read full this article<
DTI Analysis
Fractional Anisotropy (FA)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
TABLE 1
Significant Values ( P Values) for Variance of Fractional Anisotropy Identified by ANOVA Between the Groups for Each Tract
Tract Significance Genu of the corpus callosum 0.028 Left anterior corona radiata 0.178 Left anterior limb of the internal capsule 0.294 Left cingulum 0.428 Left external capsule 0.404 Left inferior longitudinal fasciculus 0.033 Left corticospinal tract 0.055 Left superior fronto-orbital fasciculus 0.835 Left superior longitudinal fasciculus 0.181 Left uncinate fasciculus 0.034 Right anterior corona radiata 0.018 Right anterior limb of the internal capsule 0.026 Right cingulum 0.906 Right external capsule 0.252 Right inferior longitudinal fasciculus 0.25 Right corticospinal tract 0.372 Right superior fronto-orbital fasciculus 0.661 Right superior longitudinal fasciculus 0.1 Right uncinate fasciculus 0.002
ANOVA, analysis of variance.
These findings demonstrate that there is clear difference between the groups in the uncinate fasciculus, genu of the corpus callosum, and the corona radiata. This analysis exemplifies structural differences between patients with neuropsychiatric systemic lupus erythematosus when compared to both healthy control patients and other patients with systemic lupus.
Get Radiology Tree app to read full this article<
Apparent Diffusion Coefficient (ADC)
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
TABLE 2
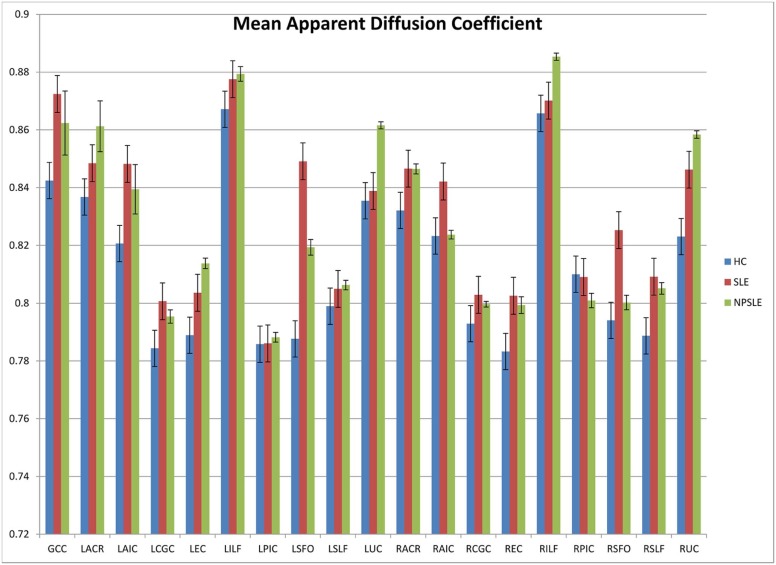
Significant Values ( P Values) for Variance of Apparent Diffusion Coefficient Identified by ANOVA Between the Groups for Each Tract
Tract Significance Genu of the corpus callosum 0.038 Left anterior corona radiata 0.461 Left anterior limb of the internal capsule 0.05 Left cingulum 0.165 Left external capsule 0.213 Left inferior longitudinal fasciculus 0.417 Left corticospinal tract 0.981 Left superior fronto-orbital fasciculus 0.005 Left superior longitudinal fasciculus 0.622 Left uncinate fasciculus 0.742 Right anterior corona radiata 0.246 Right anterior limb of the internal capsule 0.159 Right cingulum 0.328 Right external capsule 0.055 Right inferior longitudinal fasciculus 0.637 Right corticospinal tract 0.857 Right superior fronto-orbital fasciculus 0.011 Right superior longitudinal fasciculus 0.023 Right uncinate fasciculus 0.01
ANOVA, analysis of variance; HC, healthy control; NPSLE, neuropsychiatric systemic lupus erythematosus; SLE, systemic lupus erythematosus.
These findings further support differences between NPSLE patients and SLE or HC control patients within the uncinate fasciculus and genu of the corpus callosum.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Limitations and Future Directions
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
Acknowledgments
Get Radiology Tree app to read full this article<
References
1. Appenzeller S., Rondina J.M., Li L.M., et. al.: Cerebral and corpus callosum atrophy in systemic lupus erythematosus. Arthritis Rheum 2005; 52: pp. 2783-2789. Epub 2005/09/06
2. Edworthy S.M., Dobkin P.L., Clarke A.E., et. al.: Group psychotherapy reduces illness intrusiveness in systemic lupus erythematosus. J Rheumatol 2003; 30: pp. 1011-1016. Epub 2003/05/08
3. Somers E.C., Marder W., Cagnoli P., et. al.: Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol 2014; 66: pp. 369-378. Epub 2014/02/08
4. Bernatsky S., Joseph L., Pineau C.A., et. al.: A population-based assessment of systemic lupus erythematosus incidence and prevalence–results and implications of using administrative data for epidemiological studies. Rheumatology 2007; 46: pp. 1814-1818. Epub 2007/11/23
5. Sibbitt W.L., Brooks W.M., Kornfeld M., et. al.: Magnetic resonance imaging and brain histopathology in neuropsychiatric systemic lupus erythematosus. Semin Arthritis Rheum 2010; 40: pp. 32-52.
6. Ercan E., Ingo C., Tritanon O., et. al.: A multimodal MRI approach to identify and characterize microstructural brain changes in neuropsychiatric systemic lupus erythematosus. Neuroimage Clin 2015; 8: pp. 337-344.
7. Jennings J.E., Sundgren P.C., Attwood J., et. al.: Value of MRI of the brain in patients with systemic lupus erythematosus and neurologic disturbance. Neuroradiology 2004; 46: pp. 15-21.
8. Appenzeller S., Pike G.B., Clarke A.E.: Magnetic resonance imaging in the evaluation of central nervous system manifestations in systemic lupus erythematosus. Clin Rev Allergy Immunol 2008; 34: pp. 361-366.
9. Gladman D.D.: Prognosis and treatment of systemic lupus erythematosus. Curr Opin Rheumatol 1996; 8: pp. 430-437. Epub 1996/09/01
10. Kemmotsu N., Kucukboyaci N.E., Leyden K.M., et. al.: Frontolimbic brain networks predict depressive symptoms in temporal lobe epilepsy. Epilepsy Res 2014; 108: pp. 1554-1563. Epub 2014/09/17
11. Ylikoski A., Erkinjuntti T., Raininko R., et. al.: White matter hyperintensities on MRI in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke 1995; 26: pp. 1171-1177. Epub 1995/07/01
12. Cotton F., Bouffard-Vercelli J., Hermier M., et. al.: MRI of central nervous system in a series of 58 systemic lupus erythematosus (SLE) patients with or without overt neuropsychiatric manifestations. Rev Med Interne 2004; 25: pp. 8-15. Apport de l’IRM cerebrale dans une serie de 58 cas de malade lupique avec ou sans manifestations neuropsychiatriques
13. Sundgren P.C., Jennings J., Attwood J.T., et. al.: MRI and 2D-CSI MR spectroscopy of the brain in the evaluation of patients with acute onset of neuropsychiatric systemic lupus erythematosus. Neuroradiology 2005; 47: pp. 576-585.
14. Folstein M.F., Folstein S.E., McHugh P.R.: “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: pp. 189-198.
15. Cagnoli P., Harris R.E., Frechtling D., et. al.: Reduced insular glutamine and N-acetylaspartate in systemic lupus erythematosus: a single-voxel (1)H-MR spectroscopy study. Acad Radiol 2013; 20: pp. 1286-1296.
16. Wang P.I., Cagnoli P.C., McCune W.J., et. al.: Perfusion-weighted MR imaging in cerebral lupus erythematosus. Acad Radiol 2012; 19: pp. 965-970.
17. Lim K.O., Helpern J.A.: Neuropsychiatric applications of DTI—a review. NMR Biomed 2002; 15: pp. 587-593.
18. Moseley M.: Diffusion tensor imaging and aging—a review. NMR Biomed 2002; 15: pp. 553-560.
19. Jung R.E., Segall J.M., Grazioplene R.G., et. al.: Cortical thickness and subcortical gray matter reductions in neuropsychiatric systemic lupus erythematosus. PLoS ONE 2010; 5: pp. e9302. Epub 2010/03/31
20. Kozora E., Filley C.M.: Cognitive dysfunction and white matter abnormalities in systemic lupus erythematosus. J Int Neuropsychol Soc 2011; 17: pp. 385-392. Epub 2011/02/23
21. Appenzeller S., Li L.M., Costallat L.T., et. al.: Neurometabolic changes in normal white matter may predict appearance of hyperintense lesions in systemic lupus erythematosus. Lupus 2007; 16: pp. 963-971. Epub 2007/11/29
22. Schmidt-Wilcke T., Cagnoli P., Wang P., et. al.: Diminished white matter integrity in patients with systemic lupus erythematosus. Neuroimage Clin 2014; 5: pp. 291-297.
23. Bombardier C., Gladman D.D., Urowitz M.B., et. al.: Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 1992; 35: pp. 630-640. Epub 1992/06/01
24. Oishi K., Faria A., Van Zijl P., et. al.: MRI atlas of human white matter.2nd ed.2011.Academic PressAmsterdam 257 p
25. Zimny A., Szmyrka-Kaczmarek M., Szewczyk P., et. al.: In vivo evaluation of brain damage in the course of systemic lupus erythematosus using magnetic resonance spectroscopy, perfusion-weighted and diffusion-tensor imaging. Lupus 2014; 23: pp. 10-19.
26. Emmer B.J., Veer I.M., Steup-Beekman G.M., et. al.: Tract-based spatial statistics on diffusion tensor imaging in systemic lupus erythematosus reveals localized involvement of white matter tracts. Arthritis Rheum 2010; 62: pp. 3716-3721. Epub 2010/08/20
27. Bracht T., Linden D., Keedwell P.: A review of white matter microstructure alterations of pathways of the reward circuit in depression. J Affect Disord 2015; 187: pp. 45-53.
28. Harboe E., Greve O.J., Beyer M., et. al.: Fatigue is associated with cerebral white matter hyperintensities in patients with systemic lupus erythematosus. J Neurol Neurosurg Psychiatry 2008; 79: pp. 199-201.
29. Palmer S.M., Crewther S.G., Carey L.M., et. al.: A meta-analysis of changes in brain activity in clinical depression. Front Hum Neurosci 2014; 8: pp. 1045.
30. Huang X., Magder L.S., Petri M.: Predictors of incident depression in systemic lupus erythematosus. J Rheumatol 2014; 41: pp. 1823-1833. Epub 2014/08/17
31. Hughes M., Sundgren P.C., Fan X., et. al.: Diffusion tensor imaging in patients with acute onset of neuropsychiatric systemic lupus erythematosus: a prospective study of apparent diffusion coefficient, fractional anisotropy values, and eigenvalues in different regions of the brain. Acta Radiol 2007; 48: pp. 213-222.
32. Wang J.Y., Abdi H., Bakhadirov K., et. al.: A comprehensive reliability assessment of quantitative diffusion tensor tractography. Neuroimage 2012; 60: pp. 1127-1138. Epub 2012/01/10