Rationale and Objectives
Here we review the current state of multicenter radiology research (MRR), and utilize a survey of experienced researchers to identify common advantages, barriers, and resources to guide future investigators.
Materials and Methods
The Association of University Radiologists established a Radiology Research Alliance task force, Multi-center Research Studies in Radiology, composed of 12 society members to review MRR. A REDCap survey was designed to gain more insight from experienced researchers. Recipients were authors identified from a PubMed database search, utilizing search terms “multicenter” or “multisite” and “radiology.” The survey included investigator background information, reasons why, barriers to, and resources that investigators found helpful in conducting or participating in MRR.
Results
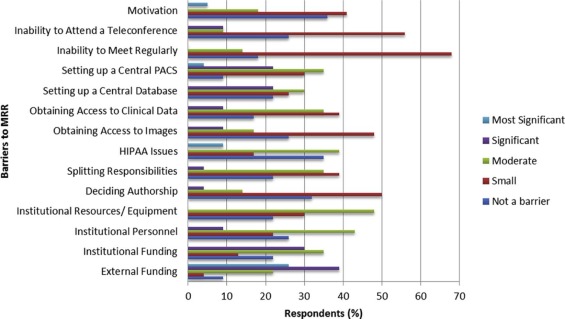
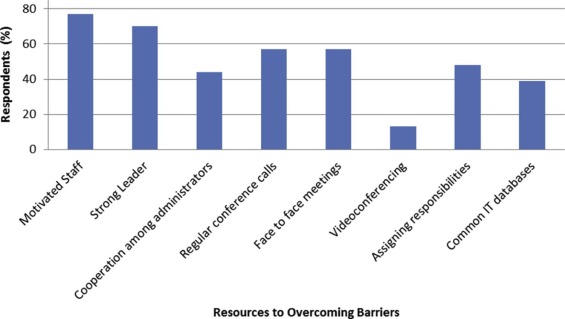
The survey was completed by 23 of 80 recipients (29%), the majority (76%) of whom served as a primary investigator on at least one MRR project. Respondents reported meeting collaborators at national or international (74%) and society (39%) meetings. The most common perceived advantages of MRR were increased sample size (100%) and improved generalizability (91%). External funding was considered the most significant barrier to MRR, reported by 26% of respondents. Institutional funding, setting up a central picture archiving and communication system, and setting up a central database were considered a significant barrier by 30%, 22%, and 22% of respondents, respectively. Resources for overcoming barriers included motivated staff (74%), strong leadership (70%), regular conference calls (57%), and at least one face-to-face meeting (57%).
Conclusions
Barriers to MRR include funding and establishing a central database and a picture archiving and communication system. Upon embarking on an MRR project, forming a motivated team who meets and speaks regularly is essential.
Introduction
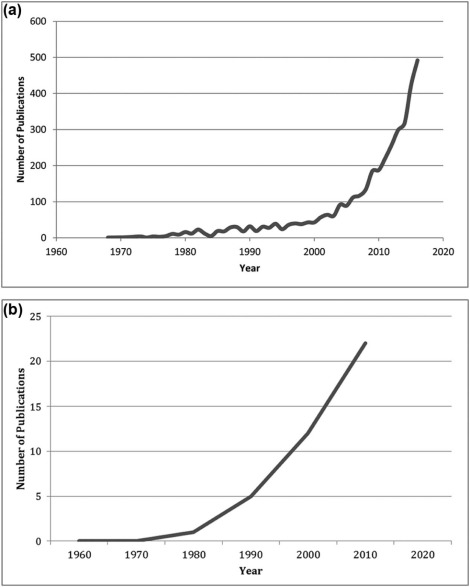
Multicenter research studies are collaborative efforts between three or more medical centers. Although two centers may allow for external validation, once extended to three centers, findings may be more widely applicable to a given population. By leveraging larger and more diverse patient populations and sharing resources, multicenter studies offer many advantages over single-institution studies . Health-care practice guidelines based on value and appropriateness criteria require the strongest available scientific evidence for their validation. Performed appropriately, multicenter research studies can provide higher-quality research data than single-institution studies . Accordingly, multicenter research trial publications over the past decade have increased dramatically in all fields of medicine ( Fig 1a ).
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and Methods
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Background Information on Investigators
Work Environment and Experience
Get Radiology Tree app to read full this article<
Primary Investigator Role
Get Radiology Tree app to read full this article<
Collaborators
Get Radiology Tree app to read full this article<
Funding Sources
Get Radiology Tree app to read full this article<
Communication
Get Radiology Tree app to read full this article<
Overall Perceived Advantages
Get Radiology Tree app to read full this article<
Potential Barriers
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Funding Sources
Get Radiology Tree app to read full this article<
Institutional Personnel and Resources
Get Radiology Tree app to read full this article<
Authorship
Get Radiology Tree app to read full this article<
Sharing Responsibilities
Get Radiology Tree app to read full this article<
Health Insurance Portability and Accountability Act (HIPAA)
Get Radiology Tree app to read full this article<
Obtaining Access to Data
Get Radiology Tree app to read full this article<
Setting Up a Central Database
Get Radiology Tree app to read full this article<
Meetings and Communication
Get Radiology Tree app to read full this article<
Motivation
Get Radiology Tree app to read full this article<
Resources to Overcome Barriers
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Imaging Performed Outside of the Main Institution
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Limitations
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
References
1. Sox H., Stern S., Owens D., et. al.: Assessment of diagnostic technology in health care: rationale, methods, problems, and directions: monograph of the council on health care technology. Washington, DC1989.
2. Atkins D., Best D., Briss P.A., et. al.: Grading quality of evidence and strength of recommendations. BMJ 2004; 328: pp. 1490.
3. Guyatt G.H., Oxman A.D., Schunemann H.J., et. al.: GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011; 64: pp. 380-382.
4. Mendelson D.S., Erickson B.J., Choy G.: Image sharing: evolving solutions in the age of interoperability. J Am Coll Radiol 2014; 11: pp. 1260-1269.
5. Mennes M., Biswal B.B., Castellanos F.X., et. al.: Making data sharing work: the FCP/INDI experience. Neuroimage 2013; 82: pp. 683-691.
6. Andriole K.P.: Security of electronic medical information and patient privacy: what you need to know. J Am Coll Radiol 2014; 11: pp. 1212-1216.
7. Kushida C.A., Nichols D.A., Jadrnicek R., et. al.: Strategies for de-identification and anonymization of electronic health record data for use in multicenter research studies. Med Care 2012; 50: pp. S82-S101.
8. Budin-Ljosne I., Isaeva J., Knoppers B.M., et. al.: Data sharing in large research consortia: experiences and recommendations from ENGAGE. Eur J Hum Genet 2014; 22: pp. 317-321.
9. Harris P.A., Taylor R., Thielke R., et. al.: Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: pp. 377-381.
10. Sprague S., Matta J.M., Bhandari M., et. al.: Multicenter collaboration in observational research: improving generalizability and efficiency. J Bone Joint Surg Am 2009; 91: pp. 80-86.
11. Hillman B.J.: ACRIN—lessons learned in conducting multi-center trials of imaging and cancer. Cancer Imaging 2005; 5: pp. S97-S101.
12. Appel L.J.: A primer on the design, conduct, and interpretation of clinical trials. Clin J Am Soc Nephrol 2006; 1: pp. 1360-1367.
13. Cook D., Brower R., Cooper J., et. al.: Multicenter clinical research in adult critical care. Crit Care Med 2002; 30: pp. 1636-1643.
14. Lindquist R., Treat-Jacobson D., Watanuki S.: A case for multisite studies in critical care. Heart Lung 2000; 29: pp. 269-277.
15. Loue S., Pike E.C.: Ethical issues in multicenter/multisite studies.Loue S.Pike E.C.Case studies in ethics and HIV research.2007.Springer USBoston, MA:pp. 175-203.
16. Corley E.A., Boardman P.C., Bozeman B.: Design and the management of multi-institutional research collaborations: theoretical implications from two case studies. Res Policy 2006; 35: pp. 975-993.
17. Irving S.Y., Curley M.A.: Challenges to conducting multicenter clinical research: ten points to consider. AACN Adv Crit Care 2008; 19: pp. 164-169.
18. Chung K.C., Song J.W.: A guide to organizing a multicenter clinical trial. Plast Reconstr Surg 2010; 126: pp. 515-523.
19. Ehrlich P.F., Newman K.D., Haase G.M., et. al.: Lessons learned from a failed multi-institutional randomized controlled study. J Pediatr Surg 2002; 37: pp. 431-436.
20. Guirro R., Ferrari Corrêa J.C.: Projeto de pesquisa multicêntrico: um desafio. Braz J Phys Ther 2011; 15: pp. v-vi.
21. Hogg R.J.: Trials and tribulations of multicenter studies. Lessons learned from the experiences of the Southwest Pediatric Nephrology Study Group (SPNSG). Pediatr Nephrol 1991; 5: pp. 348-351.
22. Maguire M.A., Gore J.C.: The current state of NIH funding of research in diagnostic radiology at U.S. medical schools. J Am Coll Radiol 2005; 2: pp. 436-443.
23. Kessel K.A., Combs S.E.: Data management, documentation and analysis systems in radiation oncology: a multi-institutional survey. Radiat Oncol 2015; 10: pp. 230.
24. Hillman B.J., Gatsonis C.: The American College of Radiology Imaging Network—clinical trials of diagnostic imaging and image-guided treatment. Semin Oncol 2008; 35: pp. 460-469.
25. Pisano E.D., Gatsonis C., Hendrick E., et. al.: Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 2005; 353: pp. 1773-1783.
26. Church T.R., Black W.C., Aberle D.R., et. al.: Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013; 368: pp. 1980-1991.
27. Aberle D.R., Chiles C., Gatsonis C., et. al.: Imaging and cancer: research strategy of the American College of Radiology Imaging Network. Radiology 2005; 235: pp. 741-751.
28. Decker S.J., Grajo J.R., Hazelton T.R., et. al.: Research challenges and opportunities for clinically oriented academic radiology departments. Acad Radiol 2016; 23: pp. 43-52.
29. Rosenkrantz A.B., Jiang A.: Associations between NIH funding and advanced bibliometric indices among radiological investigators. Acad Radiol 2016; 23: pp. 669-674.
30. Jiang A., Ginocchio L.A., Rosenkrantz A.B.: Associations between academic rank and advanced bibliometric indices among United States academic radiologists. Acad Radiol 2016; 23: pp. 1568-1572.
31. Dulhunty J.M., Boots R.J., Paratz J.D., et. al.: Determining authorship in multicenter trials: a systematic review. Acta Anaesthesiol Scand 2011; 55: pp. 1037-1043.
32. Rennie D., Yank V., Emanuel L.: When authorship fails. A proposal to make contributors accountable. JAMA 1997; 278: pp. 579-585.
33. Digiusto E.: Equity in authorship: a strategy for assigning credit when publishing. Soc Sci Med 1994; 38: pp. 55-58.
34. Whellan D.J., Ellis S.J., Kraus W.E., et. al.: Method for establishing authorship in a multicenter clinical trial. Ann Intern Med 2009; 151: pp. 414-420.