Rationale and Objectives
To evaluate the correlation between enhancement parameters of multiphase contrast-enhanced computed tomography (CT) and immunohistochemical activities of vascular endothelial growth factor (VEGF), VEGF receptors, and CD34 in hepatocellular carcinoma (HCC).
Materials and Methods
Twenty-seven patients underwent curative resection for HCC with no preoperative treatment. We defined several CT enhancement parameters by measuring attenuation values of tumor, liver parenchyma, and aorta. The stored tissue blocks were assayed for immunohistochemical activities of VEGF, two VEGF receptors (Flt-1, Flk-1), and CD34, which were correlated with the enhancement parameters of multiphase contrast-enhanced CT.
Results
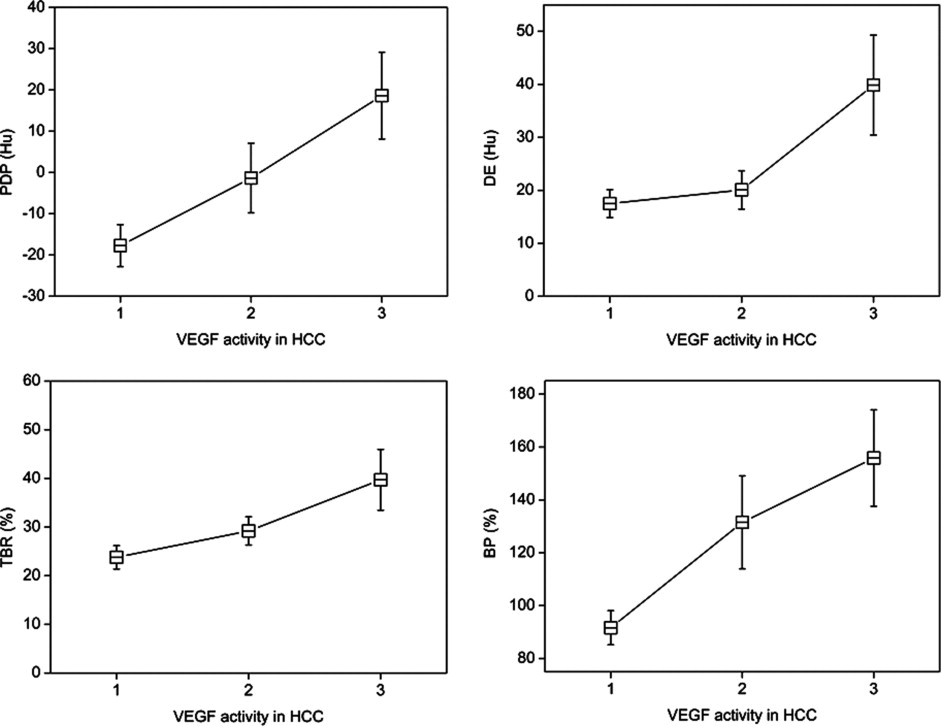
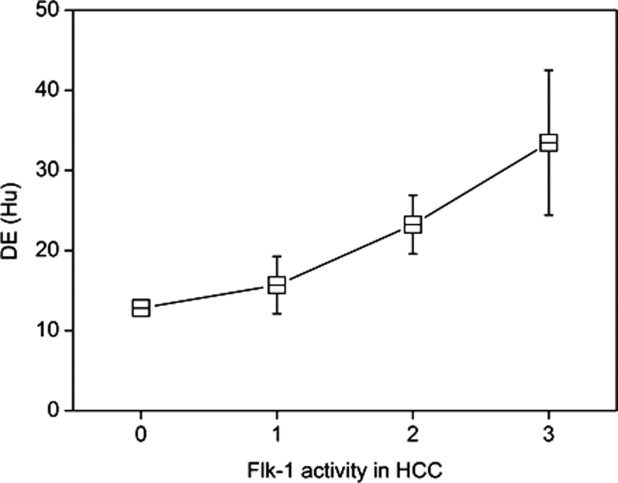
The VEGF activities in HCC showed moderate positive correlation with phase difference in portal phase, delayed enhancement (DE), tumor-blood ratio, blood pool index, and tumor-parenchyma ratio in arterial phase. The Flk-1 activities in HCC showed moderate positive correlation only with DE. CD34 activity in HCC showed positive correlation with most of the CT parameters except for DE.
Conclusion
Our study showed that several CT enhancement parameters representing mainly delayed enhancement features were well correlated with VEGF activity in HCC, and might be valuable indicators for assessing angiogenic activity in HCC.
Angiogenesis plays an important role in tumor growth and metastasis, and neovasculature facilitates shedding of tumor cells into surrounding blood vessels ( ). Growing tumors produce several angiogenic factors that stimulate endothelial cells and facilitate the formation of neovessels. Among the many angiogenic growth factors, vascular endothelial growth factor (VEGF) has been recognized as one of the most potent and effective factors ( ). VEGF expression by tumors is closely related to tumor progression and prognosis and metastasis ( ). There are currently two well-known VEGF receptors: Flt-1 and Flk-1. Flt-1 is also called VEGFR-1, and Flk-1 is also called VEGFR-2 or KDR. The expression of VEGF receptor also correlates with vascularity, metastasis, and proliferative activities of tumors ( ). Thus it stands to reason that VEGF receptors must play a role in regulating VEGF functions in the cancer microenvironment.
Hepatocellular carcinoma (HCC) is a hypervascular tumor characterized by its blood supply mainly from arterial sources ( ), and a high propensity for venous invasion. Expression of proangiogenic factors such as VEGF is known to be associated with the development of HCC ( ). CD34, a glycosylated transmembrane protein, is preferentially expressed on the surface of regenerating or migrating endothelial cells ( ), and has been investigated for localization of endothelial cells in HCC ( ). Several studies have suggested that CD34 may be a more sensitive and specific marker than other endothelial cell markers for microvessels in HCC ( ).
Get Radiology Tree app to read full this article<
Materials and methods
Patients
Get Radiology Tree app to read full this article<
Determination of CT Parameters
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Summary of CT Enhancement Parameters
CT Enhancement Parameter Definition CE CEA CT value in arterial phase – CT value in precontrast phase CEP CT value in portal phase – CT value in precontrast phase RE REA (CEA/CT value in precontrast phase) × 100 (%) REP (CEP/CT value in precontrast phase) × 100 (%) PD PDA (Tumoral CT value – parenchymal CT value) in arterial phase PDP (Tumoral CT value – parenchymal CT value) in portal phase DE Tumoral CT value in portal phase – tumoral CT value in arterial phase TBR(Tumoral peak CT value – tumoral CT value in precontrast phase)(Aortic peak CT value – aortic CT value in precontrast phase)×100(%) (Tumoral peak CT value – tumoral CT value in precontrast phase)
(Aortic peak CT value – aortic CT value in precontrast phase)
×
100
(
%
) BP (Tumoral CEP/parenchymal CEP) × 100 (%) Tumor-parenchyma ratio in arterial phase (TPA) (Tumoral CEA/parenchymal CEA) × 100 (%)
CT: computed tomography; CE: contrast-enhancement index; CEA: contrast-enhancement index in arterial phase; CEP: contrast-enhancement index in portal phase; RE: relative enhancement ratio; REA: relative enhancement ratio in arterial phase; REP: relative enhancement ratio in portal phase; PD: phase difference; PDA: phase difference in arterial phase; PDP: phase difference in portal phase.
Each of CE, RE, and PD is divided into two categories according to the phase that CT attenuation values are taken in. Labels are attached with A for arterial phase and P for portal phase. CE and RE can be measured separately in HCC and hepatic parenchyma.
Get Radiology Tree app to read full this article<
Immunohistochemical Analysis
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Tumor Size/Grade and Angiogenic Activities in HCC
Get Radiology Tree app to read full this article<
Table 2
Summary of Correlations Between Tumor Size/Grade and Immunohistochemical Activities in HCC ⁎
VEGF of HCC Flt-1 of HCC Flk-1 of HCC CD34 of HCC_r__P__r__P__r__P__r__P_ Tumor size −0.29 .15 −0.38 .051 0.20 .32 0.35 .076 Tumor grade −0.17 .39 −0.091 .65 −0.061 .76 0.35 .071
HCC: hepatocellular carcinoma; VEGF: vascular endothelial growth factor; r : correlation coefficient.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
CT Findings and Angiogenic Activities in HCC
Get Radiology Tree app to read full this article<
Table 3
Summary of the Mean Values of CE and RE for HCC and for the Liver
CEA (Hu) CEP (Hu) REA (%) REP (%) HCC 46.16 ± 25.35 69.05 ± 27.38 108.86 ± 61.49 164.19 ± 68.19 Liver 12.27 ± 10.85 58.85 ± 17.00 22.53 ± 20.98 107.03 ± 35.09
CE: contrast-enhancement index; Hu: Hounsfield unit; CEA: contrast-enhancement index in arterial phase; CEP: contrast-enhancement index in portal phase; REA: relative enhancement ratio in arterial phase; REP: relative enhancement ratio in portal phase; HCC: hepatocellular carcinoma.
Table 4
Summary of Correlations Between CT Parameters and Immunohistochemical Activities in HCC
VEGF of HCC Flt-1 of HCC Flk-1 of HCC CD34 of HCC_r__P__r__P__r__P__r__P_ CEA 0.031 .88 −0.065 .75 −0.15 .44 0.61 .001 CEP 0.28 .15 −0.17 .39 0.1 .61 0.64 .001 REA 0.071 .73 −0.058 .78 −0.15 .45 0.6 .001 REP 0.26 .19 −0.24 .22 0.058 .76 0.57 .002 PDA 0.23 .26 −0.029 .89 −0.66 .74 0.57 .002 PDP 0.43 .023 −0.12 0.54 0.24 .24 0.43 .026 DE 0.39 .047 −0.27 .18 0.4 .036 0.13 .51 TBR 0.45 .02 −0.14 .48 0.17 .39 0.54 .003 BP 0.47 .013 −0.15 .45 0.19 .34 0.4 .037 TPA 0.39 .047 −0.029 .89 −0.067 .74 0.16 .42
CT: computed tomography; VEGF: vascular endothelial growth factor; HCC: hepatocellular carcinoma; CEA: contrast-enhancement index in arterial phase; CEP: contrast-enhancement index in portal phase; REA: relative enhancement ratio in arterial phase; REP: relative enhancement ratio in portal phase; PDA: phase difference in arterial phase; PDP: phase difference in portal phase; TBR: tumor-blood ratio; TPA: tumor-parenchyma ratio in arterial phase; r : correlation coefficient.
Most CT parameters representing delayed enhancement feature are moderately correlated with VEGF activities of HCC. All CT parameters except DE are correlated with CD34 activities of HCC.
Get Radiology Tree app to read full this article<
CT Findings and Angiogenic Activities in Liver Parenchyma
Get Radiology Tree app to read full this article<
Table 5
Summary of Correlation Between CT Parameters and Immunohistochemical Activities in Liver Parenchyma
VEGF of Liver Flt-1 of Liver Flk-1 of Liver CD34 of Liver_r__P__r__P__r__P__r__P_ CEA −0.28 .16 −0.26 .20 −0.28 .16 0.064 .75 CEP −0.60 .001 −0.45 .018 −0.49 .010 −0.079 .70 REA −0.28 .16 −0.26 .20 −0.26 .19 0.039 .85 REP −0.54 .004 −0.44 .023 −0.45 .019 −0.059 .77
See Table 4 for abbreviations.
CEP and REP representing enhancement feature in portal phase are negatively correlated with VEGF, Flt-1, and Flk-1 except CD34.
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Liotta L.A., Steeg P.S., Stetler-Stevenson W.G.: Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991; 64: pp. 327-336.
2. Folkman J.: Endothelial cells and angiogenic growth factors in cancer growth and metastasis. Cancer Metastasis Rev 1990; 9: pp. 171-174.
3. Marme D.: Tumor angiogenesis: the pivotal role of vascular endothelial growth factor. World J Urol 1996; 14: pp. 166-174.
4. Takahashi Y., Kitadai Y., Bucana C.D., et. al.: Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 1995; 55: pp. 3964-3968.
5. Miura H., Miyazaki T., Kuroda M., et. al.: Increased expression of vascular endothelial growth factor in human hepatocellular carcinoma. J Hepatol 1997; 27: pp. 854-861.
6. Ueda K., Terada T., Nakamura Y., et. al.: Vascular supply in adenomatous hyperplasia of the liver and hepatocellular carcinoma: a morphometric study. Hum Pathol 1992; 23: pp. 619-626.
7. Yamaguchi R., Yano H., Iemura A., et. al.: Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology 1998; 28: pp. 68-77.
8. Schlingemann R.O., Rietveld F.J., de-Waal R.M., et. al.: Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Lab Invest 1990; 62: pp. 690-696.
9. Anthony P.P., Ramani P.: Endothelial markers in malignant vascular tumors of the liver: superiority of QB-END/10 over von Willebrand factor and Ulex europaeus agglutinin 1. J Clin Pathol 1991; 44: pp. 29-32.
10. Ruck P., Xiao J.C., Kaiserling E.: Immunoreactivity of sinusoids in hepatocellular carcinoma: an immunohistochemical study using lectin UEA-1 and antibodies against endothelial markers, including CD34. Arch Pathol Lab Med 1995; 119: pp. 173-178.
11. Tanigawa N., Lu C., Mitsui T., et. al.: Quantitation of sinusoid-like vessels in hepatocellular carcinoma: its clinical and prognostic significance. Hepatology 1997; 26: pp. 1216-1223.
12. Weidner N.: Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol 1995; 147: pp. 9-19.
13. Brasch R., Turetschek K.: MRI characterization of tumors and grading angiogenesis using macromolecular contrast media: status report. Eur J Radiol 2000; 34: pp. 148-155.
14. Kanematsu M., Osada S., Amaoka N., et. al.: Expression of vascular endothelial growth factor in hepatocellular carcinoma and the surrounding liver: correlation with angiographically assisted CT. AJR Am J Roentgenol 2004; 183: pp. 1585-1593.
15. Wang B., Gao Z.Q., Yan X.: Correlative study of angiogenesis and dynamic contrast-enhanced magnetic resonance imaging features of hepatocellular carcinoma. Acta Radiol 2005; 46: pp. 353-358.
16. Wang J.H., Min P.Q., Wang P.J., et. al.: Dynamic CT evaluation of tumor vascularity in renal cell carcinoma. AJR Am J Roentgenol 2006; 186: pp. 1423-1430.
17. Thompson W.D., Li W.W., Maragoudakis M.: The clinical manipulation of angiogenesis: pathology, side-effects, surprises and opportunities with novel human therapies. J Pathol 2000; 190: pp. 330-337.
18. Patt Y.Z., Hassan M.M., Lozano R.D., et. al.: Durable clinical response of refractory hepatocellular carcinoma to orally administered thalidomide. Am J Clin Oncol 2000; 23: pp. 319-321.
19. Gasparini G., Weidner N., Bevilacqua P., et. al.: Tumor microvessel density, p53 expression, tumor size, and peritumoral lymphatic vessel invasion are relevant prognostic markers in node-negative breast carcinoma. J Clin Oncol 1994; 12: pp. 454-466.
20. Cuenod C.A., Fournier L., Balvay D., et. al.: Tumor angiogenesis: pathophysiology and implications for contrast-enhanced MRI and CT assessment. Abdom Imaging 2006; 31: pp. 188-193.
21. Padhani A.R.: Functional MRI for anticancer therapy assessment. Eur J Cancer 2002; 38: pp. 2116-2127.
22. Miles K.A.: Functional computed tomography in oncology. Eur J Cancer 2002; 38: pp. 2079-2084.
23. Cuenod C., Leconte I., Siauve N., et. al.: Early changes in liver perfusion caused by occult metastases in rats: detection with quantitative CT. Radiology 2001; 218: pp. 556-561.
24. Poon R.T., Ng I.O., Lau C., et. al.: Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol 2002; 20: pp. 1775-1785.
25. Kwak B.K., Shim H.J., Park E.S., et. al.: Hepatocellular carcinoma: correlation between vascular endothelial growth factor level and degree of enhancement by multiphase contrast-enhanced computed tomography. Invest Radiol 2001; 36: pp. 487-492.
26. Jeng K.S., Sheen I.S., Wang Y.C., et. al.: Is the vascular endothelial growth factor messenger RNA expression in resectable hepatocellular carcinoma of prognostic value after resection?. World J Gastroenterol 2004; 10: pp. 676-681.
27. Ng I.O., Poon R.T., Lee J.M., et. al.: Microvessel density, vascular endothelial growth factor and its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am J Clin Pathol 2001; 116: pp. 838-845.
28. Buckley D.L.: Uncertainty in the analysis of tracer kinetics using dynamic contrast-enhanced T1-weighted MRI. Magn Reson Med 2002; 47: pp. 601-606.
29. Padhani A.R., Neeman M.: Challenges for imaging angiogenesis. Br J Radiol 2001; 74: pp. 886-890.
30. Anderson H., Price P., Blomley M., et. al.: Measuring changes in human tumour vasculature in response to therapy using functional imaging techniques. Br J Cancer 2001; 85: pp. 1085-1093.
31. Tajima T., Honda H., Taguchi K., et. al.: Sequential hemodynamic change in hepatocellular carcinoma and dysplastic nodules. AJR Am J Roentgenol 2002; 178: pp. 885-897.
32. Leggett D.A., Kelley B.B., Bunce I.H., et. al.: Colorectal cancer: diagnostic potential of CT measurements of hepatic perfusion and implications for contrast enhancement protocols. Radiology 1997; 205: pp. 716-720.
33. Fournier L.S., Cuenod C.A., de Bazelaire C., et. al.: Early modifications of hepatic perfusion measured by functional CT in a rat model of hepatocellular carcinoma using a blood pool contrast agent. Eur Radiol 2004; 14: pp. 2125-2133.
34. Van Beers B.E., Leconte I., Materne R., et. al.: Hepatic perfusion parameters in chronic liver disease: dynamic CT measurements correlated with disease severity. AJR Am J Roentgenol 2001; 176: pp. 667-673.
35. Zeng H., Sanyal S., Mukhopadhyay D.: Tyrosine residues 951 and 1059 of vascular endothelial growth factor receptor-2 (KDR) are essential for vascular permeability factor/vascular endothelial growth factor-induced endothelium migration and proliferation, respectively. J Biol Chem 2001; 276: pp. 32714-32719.
36. Heidenreich R., Machein M., Nicolaus A., et. al.: Inhibition of solid tumor growth by gene transfer of VEGF receptor-1 mutants. Int J Cancer 2004; 111: pp. 348-357.
37. Plate K.H., Breier G., Weich H.A., et. al.: Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer 1994; 59: pp. 520-529.
38. de Jong J.S., van Diest P.J., van der Valk P., et. al.: Expression of growth factors, growth inhibiting factors, and their receptors in invasive breast cancer. J Pathol 1998; 184: pp. 44-52.