Rationale and Objectives
An animal model of partial hepatic ischemia/reperfusion injury (I/R) has benefits for decision making and clinical management after liver transplantation or massive hepatic resection. The aim of this study was to evaluate the change in perfusion parameters after partial hepatic I/R in rabbits using multislice computed tomography perfusion imaging.
Materials and Methods
Thirty rabbits underwent 60 minutes of left hepatic lobar ischemia followed by 0.5, 2, 6, 12, and 24 hours of reperfusion (six rabbits were used for each reperfusion interval). An additional six rabbits served as sham-operated controls. The perfusion indices of hepatic arterial perfusion, hepatic portal perfusion, total liver perfusion, and hepatic perfusion index were measured. Levels of serum aspartate transaminase and alanine transaminase and liver histopathology at different time points were also examined.
Results
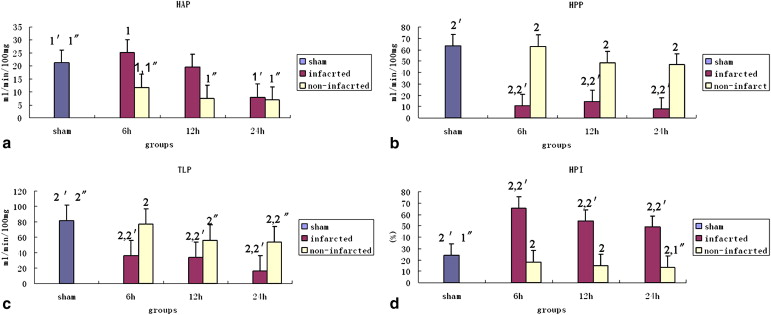
Hepatic microvascular flow patterns showed heterogeneity in the 6-hour, 12-hour, and 24-hour groups. Computed tomographic perfusion parameters were significantly different between infarcted liver tissue and viable liver tissue. In poorly enhancing tissues in the 6-hour, 12-hour, and 24-hour groups, hepatic portal perfusion and total liver perfusion were lower compared to the sham group, but hepatic arterial perfusion of poorly enhancing tissues significantly increased in the 6-hour group and then decreased slightly from 12 to 24 hours after reperfusion. The hepatic perfusion index was always higher compared to that of the sham group. Hepatic arterial perfusion, hepatic portal perfusion, total liver perfusion, and hepatic perfusion index in the noninfarcted areas decreased slowly from 6 to 24 hours after reperfusion. The levels of alanine transaminase and aspartate transaminase in the I/R groups significantly increased after reperfusion and were correlated with the computed tomographic perfusion indices of infarcted liver tissue.
Conclusions
Computed tomographic perfusion can dynamically monitor the pathologic processes of liver I/R and reveal the underlying microvascular disorder, improving clinical management after liver surgery.
Ischemia/reperfusion injury (I/R) in the liver is a critical factor affecting organ and patient survival, especially after massive hepatic resection or liver transplantation. I/R can cause cellular injury and induce cell death, as well as cause acute organ failure and chronic rejection after liver transplantation, and is therefore associated with high morbidity and mortality . Accumulating evidence suggests that microcirculatory disturbances and capillary perfusion failure, subsequent to I/R, are major factors in the pathogenesis of I/R-mediated liver injury. Heterogeneity of hepatic microvascular perfusion is more pervasive, and blood flow within individual sinusoids may become completely blocked .
Although serum levels of aspartate transaminase (AST) and alanine transaminase (ALT) activities are used to assess the degree of liver function loss, they do not offer spatial information regarding liver injury . It is important to further localize infarcted tissues and functional properties of the liver as well as vascular and extravascular complications (quantification and localization of damaged liver tissue). Therefore, the ability to quantitatively assess hemodynamic changes in the capillary network in hepatic I/R has become a major challenge for radiologists.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Animals
Get Radiology Tree app to read full this article<
Surgical Procedures and Experimental Groups
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Imaging
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Image Analysis
Get Radiology Tree app to read full this article<
Liver Enzyme Chemistry and Liver Tissue Histology
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Assessment of Liver Damage by Serum Activities
Get Radiology Tree app to read full this article<
Table 1
Levels of ALT and AST in Sham and I/R Groups
Group ALT (U/L) AST (U/L) Sham 84 ± 4.8 83 ± 5.4 0.5 hour 136 ± 19.7 436 ± 70.7 2 hour 326 ± 58.1 684 ± 90.4 6 hour 451 ± 25.0 1013 ± 86.4 12 hour 378 ± 61.7 1176 ± 73.8 24 hour 351 ± 32.5 1205 ± 97.3
Data are expressed as mean ± standard deviation.
ALT, alanine transaminase; AST, aspartate transaminase; I/R, ischemia/reperfusion.
Get Radiology Tree app to read full this article<
Characteristics of CT Perfusion Map and Perfusion Parameter Changes
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 2
Comparison of Perfusion Parameters in the Sham and I/R Groups
Group Perfusion Parameter HAP (mL/min/100 mg) HPP (mL/min/100 mg) TLP (mL/min/100 mg) HPI (%) Sham 21 ± 10.5 64 ± 24.0 81 ± 24.8 24 ± 7.5 0.5 hour 9 ± 1.2 † 15 ± 1.7 † 26 ± 5.6 † 48 ± 7.2 † 2 hour 53 ± 10.7 † 16 ± 2.7 † 69 ± 8.2 76 ± 6.9 † 6 hour 18 ± 5.3 37 ± 9.0 † 56 ± 9.4 † 42 ± 5.2 † 12 hour 14 ± 7.0 31 ± 11.7 † 45 ± 13.1 † 35 ± 11.1 ∗ 24 hour 7 ± 1.4 † 27 ± 11.1 † 35 ± 11.6 † 31 ± 6.9
Data are expressed as mean ± standard deviation. TLP = HAP + HPP; HPI = HAP/HAP + HPP. One-way analysis of variance was applied to assess significant differences among the perfusion parameters. The results for the 6-hour, 12-hour, and 24-hour groups were the average values of the infarcted and noninfarcted liver tissues for each corresponding model of I/R. For all groups, n = 6.
HAP, hepatic arterial perfusion; HPI, hepatic perfusion index; HPP, hepatic portal perfusion; I/R, ischemia/reperfusion; TLP, total liver perfusion.
∗ P < .05 and † P < .01 versus sham group.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Correlations
Get Radiology Tree app to read full this article<
Table 3
Correlations Between Perfusion Parameters and AST and ALT in Nonenhancing Necrotic Tissue in the 6-Hour, 12-Hour, and 24-Hour Groups
Lack of Enhancement of Necrotic Tissue HAP HPP TLP HPI_r__P__r__P__r__P__r__P_ AST −0.16 .46 −0.81 .00 −0.75 .00 0.75 .00 ALT −0.31 .14 −0.49 .13 −0.54 .01 0.42 .04
ALT, alanine transaminase; AST, aspartate transaminase; HAP, hepatic arterial perfusion; HPI, hepatic perfusion index; HPP, hepatic portal perfusion; TLP, total liver perfusion.
Get Radiology Tree app to read full this article<
Histologic Findings
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Okaya T., Lentsch A.B.: Cytokine cascades and the hepatic inflammatory response to ischemia and reperfusion. J Invest Surg 2003; 16: pp. 141-147.
2. Polat E., Topaloglu S., Sokmensuer C., et. al.: Heterogeneity of damage between segments of rat liver after inflow-outflow obstruction. Transplant Proc 2006; 38: pp. 3075-3081.
3. Serracino-Inglott F., Habib N.A., Mathie R.T.: Hepatic ischemia-reperfusion injury. Am J Surg 2001; 181: pp. 160-166.
4. Terajima H., Kondo T., Enders G., et. al.: Reduction of hepatic microcirculatory failure caused by normothermic ischemia/reperfusion-induced injury by means of heat shock preconditioning. Shock 1999; 12: pp. 329-334.
5. Kim N., Cao W., Song I.S., et. al.: Distinct kinases are involved in contraction of cat esophageal and lower esophageal sphincter smooth muscles. Am J Physiol Cell Physiol 2004; 287: pp. C384-C394.
6. van Werkhoven J.M., Schuijf J.D., Gaemperli O., et. al.: Prognostic value of multislice computed tomography and gated single-photon emission computed tomography in patients with suspected coronary artery disease. J Am Coll Cardiol 2009; 53: pp. 623-632.
7. Miles K.A.: Perfusion CT for the assessment of tumour vascularity: which protocol?. Br J Radiol 2003; 76: pp. S36-S42.
8. Taha M.O., Goncalves P.F., Vidigal R.O., et. al.: Protective effects of heparin on hepatic ischemia and reperfusion lesions in rabbits. Transplant Proc 2009; 41: pp. 812-815.
9. Wu X., Wang H., Chen F., et. al.: Rat model of reperfused partial liver infarction: characterization with multiparametric magnetic resonance imaging, microangiography, and histomorphology. Acta Radiol 2009; 50: pp. 276-287.
10. Cheung J.S., Fan S.J., Chow A.M., et. al.: In vivo DTI assessment of hepatic ischemia reperfusion injury in an experimental rat model. J Magn Reson Imaging 2009; 30: pp. 890-895.
11. Hirakawa A., Takeyama N., Nakatani T., et. al.: Mitochondrial permeability transition and cytochrome c release in ischemia-reperfusion injury of the rat liver. J Surg Res 2003; 111: pp. 240-247.
12. Muller J.M., Vollmar B., Menger M.D.: Pentoxifylline reduces venular leukocyte adherence (“reflow paradox”) but not microvascular “no reflow” in hepatic ischemia/reperfusion. J Surg Res 1997; 71: pp. 1-6.
13. van Nieuw Amerongen G.P., van Hinsbergh V.W.: Targets for pharmacological intervention of endothelial hyperpermeability and barrier function. Vasc Pharmacol 2002; 39: pp. 257-272.
14. Martinez-Mier G., Toledo-Pereyra L.H., McDuffie J.E., et. al.: Neutrophil depletion and chemokine response after liver ischemia and reperfusion. J Invest Surg 2001; 14: pp. 99-107.
15. Okajima K., Harada N., Uchiba M., et. al.: Neutrophil elastase contributes to the development of ischemia-reperfusion-induced liver injury by decreasing endothelial production of prostacyclin in rats. Am J Physiol Gastrointest Liver Physiol 2004; 287: pp. G1116-G1123.
16. Taha M.O., Simoes M.J., Noguerol E.C., et. al.: Effects of allopurinol on ischemia and reperfusion in rabbit livers. Transplant Proc 2009; 41: pp. 820-823.
17. Clemens M.G., Zhang J.X.: Regulation of sinusoidal perfusion: in vivo methodology and control by endothelins. Semin Liver Dis 1999; 19: pp. 383-396.
18. Garcia-Pagan J.C., Zhang J.X., Sonin N., et. al.: Ischemia/reperfusion induces an increase in the hepatic portal vasoconstrictive response to endothelin-1. Shock 1999; 11: pp. 325-329.
19. Ling Y.Q., Shibamoto T., Honda T., et. al.: Increased sinusoidal pressure is associated with early liver weight gain in ischemia-reperfusion injury in isolated perfused rat liver. J Surg Res 2000; 88: pp. 70-77.
20. Zulke C., Anthuber M., Kramling H.J., et. al.: Primary shunt perfusion detected by colour flow Doppler imaging and its impact on liver allograft survival. Clin Transplant 1997; 11: pp. 163-168.
21. Richter S., Vollmar B., Mucke I., et. al.: Hepatic arteriolo-portal venular shunting guarantees maintenance of nutritional microvascular supply in hepatic arterial buffer response of rat livers. J Physiol 2001; 531: pp. 193-201.
22. Peralta C., Bartrons R., Riera L., et. al.: Hepatic preconditioning preserves energy metabolism during sustained ischemia. Am J Physiol Gastrointest Liver Physiol 2000; 279: pp. G163-G171.
23. Zimmerman M.A., Ghobrial R.M.: When shouldn’t we retransplant?. Liver Transpl 2005; 11: pp. S14-S20.
24. Shirai S., Sato M., Suwa K., et. al.: Single photon emission computed tomography-based three-dimensional conformal radiotherapy for hepatocellular carcinoma with portal vein tumor thrombus. Int J Radiat Oncol Biol Phys 2009; 73: pp. 824-831.
![Figure 1, Computed tomographic perfusion maps of hepatic arterial perfusion (HAP), hepatic portal perfusion (HPP), and hepatic perfusion index HPI (rain map). The 0.5-hour group (b) showed a lack of perfusion pattern, with obvious declines in HAP, HPP, and total liver perfusion (TLP), compared to the sham group (a) . (c) HAP and HPI increased significantly after 2 hours of reperfusion as well as a moderate rise in HPP. (d) The low-enhancement area (regions of interest [ROIs] T4–T6) can be distinguished from the ischemia-induced left lobe in the 6-hour group. The increase in HAP and decrease in HPP in the low-enhancement area (ROIs T4–T6) contrast with the enhancement of viable liver tissue (ROIs T1–T3), implying an “arterial buffer response,” and are also an indicator of deficient blood supply to the liver parenchyma. (e) As depicted in the 24-hour group, HAP, HPP, and TLP significantly decreased in both poorly enhancing and viable enhancing liver tissues, implying progressive infarction of liver tissue. HU, Hounsfield units.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/PerfusionComputedTomographyEvaluationofPartialHepaticIschemiaReperfusioninaRabbitModel/0_1s20S1076633211002613.jpg)
![Figure 3, Pathologic samples from ischemia/reperfusion experimental groups. (a) The 0.5-hour group showed diffuse swelling of hepatocytes with intracellular edema, with collections of erythrocytes depositing in sinusoids and intravascular hemoconcentration in the central vein using hematoxylin and eosin staining. (b) The 2-hour group showed clusters of neutrophils accumulating in portal areas (portal vein [PV] and bile ducts [BD]). (c) The 12-hour group was characterized by patchy distribution of degenerative hepatocytes with apparent edema in postischemic liver tissue and increasing neutrophils encountered throughout the liver and extravasating into hepatic parenchyma. (d) The 24-hour group showed disorganized structures of atrophic hepatocytes, collapsed sinusoids, and shrunken central vein together with hepatic artery (HA) dilatation in the noninfarcted liver tissues. (e) Focal coagulative necroses accompanied by the presence of moderate numbers of neutrophils at the periphery of the infarcted lobules.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/PerfusionComputedTomographyEvaluationofPartialHepaticIschemiaReperfusioninaRabbitModel/2_1s20S1076633211002613.jpg)