Rationale and Objectives
It was hypothesized that perfusion computed tomography (CT), blood flow (BF), blood volume (BV), and vascular permeability surface area (PS) product parameters would be predictive of therapeutic anticancer agent uptake in pancreatic cancer, facilitating image-guided interpretation of human treatments. The hypothesis was tested in an orthotopic rabbit model of pancreatic cancer, by establishing the model, imaging with endoscopic ultrasound (EUS) and contrast CT, and spatially comparing the perfusion maps to the ex vivo uptake values of the injected photosensitizer, verteporfin.
Materials and Methods
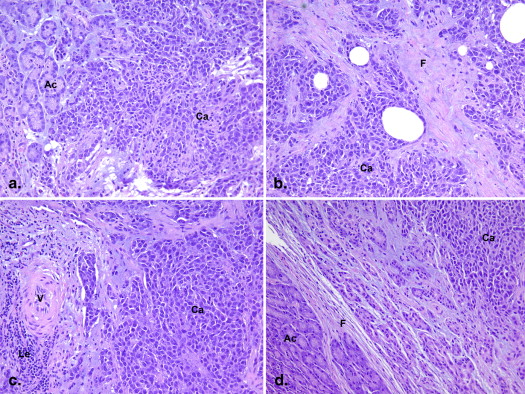
Nine New Zealand white rabbits underwent direct pancreas implantation of VX2 tumors, and CT perfusion or EUS was performed 10 days postimplantation. Verteporfin was injected during CT imaging, and the tissue was removed 1 hour postinjection for frozen tissue fluorescence scanning. Region-of-interest comparisons of CT data with ex vivo fluorescence and histopathologic staining were performed.
Results
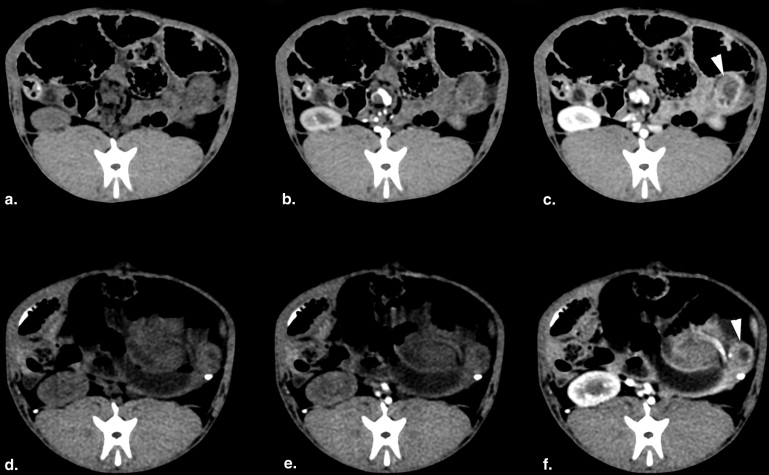
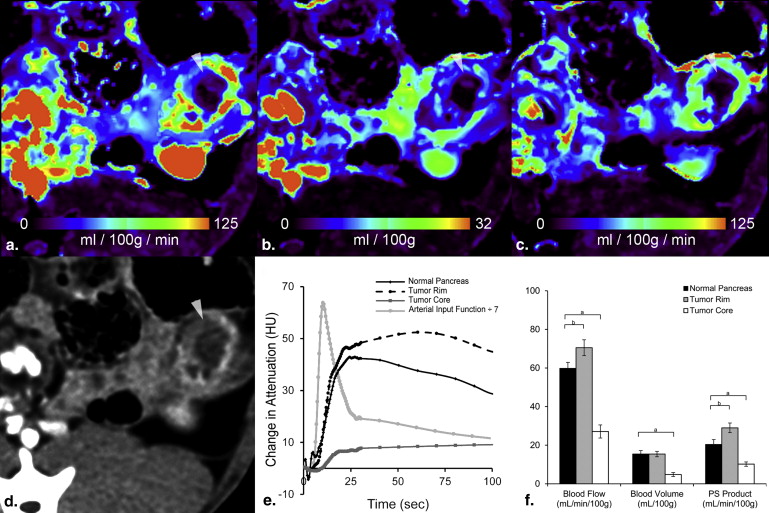
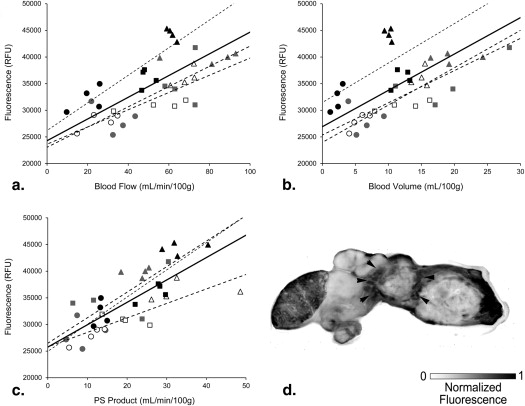
Dynamic contrast-enhanced CT showed enhanced BF, BV, and PS in the tumor rim and decreased BF, BV, and PS in the tumor core. Significant correlations were found between ex vivo verteporfin concentration and each of BF, BV, and PS.
Conclusions
The efficacy of verteporfin delivery in tumors is estimated by perfusion CT, providing a noninvasive method of mapping photosensitizer dose.
The 5-year survival of pancreatic adenocarcinoma is approximately 3%, among the most dismal prognoses in all of oncology . Radical resection with or without adjuvant or neoadjuvant treatment is the only means of improving long-term survival but is only an option in approximately 25% of patients . For locally advanced and unresectable disease, a main focus of patient management is the preservation of an acceptable quality of life for as long as possible through palliative therapy . Treatments with minimal side effects that are able to provide some degree of tumor control are preferred in these cases, but options are currently limited. New therapeutic options—including photodynamic therapy (PDT) , radiofrequency ablation , tumor vaccines , and targeted nanoparticles —may be treatment options for unresectable pancreatic cancer, and the advance of suitable preclinical animal models will be critical to the development and implementation of each to accurately assess their efficacy before widespread clinical translation. For example, in a recent phase I/II study, verteporfin-based PDT was delivered to locally advanced pancreatic cancer . Although PDT was clearly able to induce a dose–dependent damage to the cancers, it was also noted that the treatment induced a necrotic volume that was inversely related to the observed contrast computed tomography (CT) enhancement . Beyond this, it is well known that drug permeability in cancer is directly related to perfusion in a number of cancer and drug combinations , and so, perfusion imaging is a reasonable way to diagnostically assess the penetration of therapeutics into the lesion. The goal of this study was to use a rabbit orthotopic model of cancer in the pancreas, and use it with conventional ultrasound and CT imaging to characterize the uptake and dose delivery of verteporfin to better understand the role of this type of imaging in human PDT treatments.
PDT is an alternative treatment for cancer, which uses the interaction of light and a photosensitizer, in the presence of oxygen, to produce singlet oxygen . The singlet oxygen causes direct cell death through necrosis , as well as indirect cell death by vascular damage–induced hypoxia. A major benefit of PDT is that it exerts its effect photochemically, rather than thermally; so, connective tissues such as collagen and elastin remain intact, preserving the mechanical integrity of the tissue . The photosensitizer drug, such as verteporfin used in this study, is most often delivered intravenously. Localized tissue necrosis can be produced by delivering light from a low-power laser directly to the tumor through a small diameter fiber . Because both drug delivery and light delivery affect the volume of tumor necrosis, dynamic CT imaging is proposed for planning and evaluating PDT treatment in pancreatic cancer.
Get Radiology Tree app to read full this article<
Materials and methods
Animal Experiment
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Image Acquisition
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Computed Tomography Analysis
Get Radiology Tree app to read full this article<
Ex Vivo Fluorescence Imaging and Histopathology
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
Acknowledgments
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Warshaw A.L., Fernandez-del Castillo C.: Pancreatic carcinoma. N Engl J Med 1992; 326: pp. 455-465.
2. Gillen S., Schuster T., Meyer Zum Buschenfelde C., et. al.: Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010; 7: pp. e1000267.
3. Li D., Xie K., Wolff R., et. al.: Pancreatic cancer. Lancet 2004; 363: pp. 1049-1057.
4. Dolmans D.E., Fukumura D., Jain R.K.: Photodynamic therapy for cancer. Nat Rev Cancer 2003; 3: pp. 380-387.
5. Bown S.G., Rogowska A.Z., Whitelaw D.E., et. al.: Photodynamic therapy for cancer of the pancreas. Gut 2002; 50: pp. 549-557.
6. Girelli R., Frigerio I., Salvia R., et. al.: Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg 2010; 97: pp. 220-225.
7. Jaffee E.M., Hruban R.H., Biedrzycki B., et. al.: Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 2001; 19: pp. 145-156.
8. Weissleder R., Kelly K., Sun E.Y., et. al.: Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol 2005; 23: pp. 1418-1423.
9. Huggett M.T., Jermyn M., Gillams A., et. al.: Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br J Cancer 2014; 110: pp. 1698-1704.
10. Jermyn M., Davis S.C., Dehghani H., et. al.: CT contrast predicts pancreatic cancer treatment response to verteporfin-based photodynamic therapy. Phys Med Biol 2014; 59: pp. 1911-1921.
11. Stapleton S., Allen C., Pintilie M., et. al.: Tumor perfusion imaging predicts the intra-tumoral accumulation of liposomes. J Control Release 2013; 172: pp. 351-357.
12. Gaba R.C., Jin B., Wang D., et. al.: Locoregional chemoembolic delivery: prediction with transcatheter intraarterial perfusion MRI. AJR Am J Roentgenol 2012; 198: pp. 1196-1202.
13. Wilson B.C., Patterson M.S.: The physics of photodynamic therapy. Phys Med Biol 1986; 31: pp. 327-360.
14. Weishaupt K.R., Gomer C.J., Dougherty T.J.: Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res 1976; 36: pp. 2326-2329.
15. Barr H., Tralau C.J., Boulos P.B., et. al.: The contrasting mechanisms of colonic collagen damage between photodynamic therapy and thermal injury. Photochem Photobiol 1987; 46: pp. 795-800.
16. Pascal J., Bearer E.L., Wang Z., et. al.: Mechanistic patient-specific predictive correlation of tumor drug response with microenvironment and perfusion measurements. Proc Natl Acad Sci U S A 2013; 110: pp. 14266-14271.
17. Nagakawa T., Kayahara M., Ohta T., et. al.: Patterns of neural and plexus invasion of human pancreatic cancer and experimental cancer. Int J Pancreatol 1991; 10: pp. 113-119.
18. Eifler A.C., Lewandowski R.J., Virmani S., et. al.: Development of a VX2 pancreatic cancer model in rabbits: a pilot study. J Vasc Interv Radiol 2009; 20: pp. 1075-1082.
19. Gunn J, Tichauer KM, Moodie K, et al. Pancreas tumor model in rabbit imaged by perfusion CT scans. In: Proc of SPIE. San Francisco, CA, 2013; 85681E-885681E.
20. Purdie T.G., Henderson E., Lee T.Y.: Functional CT imaging of angiogenesis in rabbit VX2 soft-tissue tumour. Phys Med Biol 2001; 46: pp. 3161-3175.
21. St Lawrence K.S., Lee T.Y.: An adiabatic approximation to the tissue homogeneity model for water exchange in the brain: I. Theoretical derivation. J Cereb Blood Flow Metab 1998; 18: pp. 1365-1377.
22. Naish J.H., Kershaw L.E., Buckley D.L., et. al.: Modeling of contrast agent kinetics in the lung using T1-weighted dynamic contrast-enhanced MRI. Magn Reson Med 2009; 61: pp. 1507-1514.
23. Murphy B.D., Fox A.J., Lee D.H., et. al.: Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke 2006; 37: pp. 1771-1777.
24. Zeger S.L., Qaqish B.: Markov regression models for time series: a quasi-likelihood approach. Biometrics 1988; 44: pp. 1019-1031.
25. Isabelle M, O’Hara JA, Samkoe K, et al. In vivo sampling of verteporfin uptake in pancreas cancer xenograft models: comparison of surface, oral, and interstitial measurements. In: Proc. SPIE: SPIE, 2010; 755116.
26. Isabelle M., Davis S.C., Li Z., et. al.: Tissue photosensitizer dosimetry using spectrally-resolved fluorescence for pre-clinical and clinical verteporfin-PDT of pancreatic cancer.Proc. SPIE: SPIE.2012.pp. 821015.
27. Forkman J.: Estimator and tests for common coefficients of variation in normal distributions. Commun Stat Theory Methods 2009; 38: pp. 233-251.
28. Ng Q.S., Thng C.H., Lim W.T., et. al.: Dynamic contrast-enhanced computed tomography in metastatic nasopharyngeal carcinoma: reproducibility analysis and observer variability of the distributed parameter model. Invest Radiol 2012; 47: pp. 5-10.
29. Xu J., Liang Z., Hao S., et. al.: Pancreatic adenocarcinoma: dynamic 64-slice helical CT with perfusion imaging. Abdom Imaging 2009; 34: pp. 759-766.
30. Lu N., Feng X.Y., Hao S.J., et. al.: 64-slice CT perfusion imaging of pancreatic adenocarcinoma and mass-forming chronic pancreatitis. Acad Radiol 2011; 18: pp. 81-88.
31. Delrue L., Blanckaert P., Mertens D., et. al.: Tissue perfusion in pathologies of the pancreas: assessment using 128-slice computed tomography. Abdom Imaging 2012; 37: pp. 595-601.
32. Samkoe K.S., Bryant A., Gunn J.R., et. al.: Contrast enhanced-magnetic resonance imaging as a surrogate to map verteporfin delivery in photodynamic therapy. J Biomed Opt 2013; 18: pp. 120504.
33. Luo W., Zhou X., Zheng X., et. al.: Role of sonography for implantation and sequential evaluation of a VX2 rabbit liver tumor model. J Ultrasound Med 2010; 29: pp. 51-60.
34. Warren K.W., Cattell R.B., Blackburn J.P., et. al.: A long-term appraisal of pancreaticoduodenal resection for peri-ampullary carcinoma. Ann Surg 1962; 155: pp. 653-662.
35. Hingorani S.R., Wang L., Multani A.S., et. al.: Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005; 7: pp. 469-483.
36. Cubilla A.L., Fitzgerald P.J., Fortner J.G.: Pancreas cancer—duct cell adenocarcinoma: survival in relation to site, size, stage and type of therapy. J Surg Oncol 1978; 10: pp. 465-482.