Positron emission tomography (PET), commonly performed in conjunction with computed tomography (CT), has revolutionized oncologic imaging. PET/CT has become the standard of care for the initial staging and assessment of treatment response for many different malignancies. Despite this success, PET/CT is often supplemented by magnetic resonance imaging (MRI), which offers superior soft-tissue contrast and a means of assessing cellular density with diffusion-weighted imaging. Consequently, PET/MRI, the newest clinical hybrid imaging modality, has the potential to provide added value over PET/CT or MRI alone. The purpose of this article is to provide a comprehensive review of the current body of literature pertaining to the clinical performance of PET/MRI, with the aim of summarizing current evidence and identifying gaps in knowledge to direct clinical expansion and future research. Multiple example cases are also provided to illustrate the central findings of these publications.
Introduction
Positron emission tomography (PET) has revolutionized the imaging evaluation of numerous oncologic conditions by exploiting biochemical and physiologic differences between tumor cells and normal tissues . Often performed in conjunction with computed tomography (CT), PET utilizing the glucose analog 2-deoxy-2-[ 18 F]fluoro-D-glucose (FDG) has become the standard of care for the initial staging and the subsequent assessment of treatment response for many malignancies . Tumor uptake of FDG reflects the increased rates of aerobic glycolysis that occur in many cancer cells (the Warburg effect) relative to most normal tissues and benign lesions. The resulting distribution of FDG thereby allows for anatomic delineation of local and distant tumor spread by PET/CT and provides a measure of a key aspect of cancer metabolism. Many PET tracers have also been developed to take advantage of other distinctive tumor properties, such as elevated amino acid transport or altered receptor expression .
Despite its proven utility, FDG-PET/CT has important limitations, especially with respect to local tumor staging and the characterization of certain incidental lesions. In such situations, further evaluation with magnetic resonance imaging (MRI) may be indicated to achieve optimal clinical management. The superb soft-tissue contrast of MRI and its capacity to assess cellular density by diffusion-weighted imaging (DWI) constitute powerful supplements to the molecular and metabolic data of PET. Consequently, PET/MRI, the newest clinical hybrid imaging modality, has significant potential to improve the diagnosis, initial staging, and subsequent restaging of numerous cancers. However, studies demonstrating such benefits are needed to support the routine clinical use of PET/MRI, particularly to justify the added expense and complexity of PET/MRI instead of PET/CT. This review aims to summarize the current body of evidence in support of PET/MRI, as well as current challenges and gaps in knowledge, and to identify oncologic conditions likely to benefit from its clinical use. We also present case examples to illustrate specific advantages of PET/MRI. Overall, this article should familiarize the reader with the current clinical applications of PET/MRI in oncology and provide an overview of the specific scenarios in which PET/MRI may provide added value over PET/CT or MRI alone.
Current Challenges
Technical Considerations
Before delving into the clinical evidence, it is essential to discuss briefly the technical development of PET/MRI, so as to understand some of the inherent advantages, challenges, and limitations. The earliest approach to combining PET and MRI data was through software fusion of PET or PET/CT images with separately acquired MRI. The first combined apparatuses were sequential PET/MRI systems that consisted of individual PET and MRI elements connected by a common table. The newer integrated PET/MRI systems acquire PET and MRI data simultaneously in the same bore. This latter strategy may improve scanning efficiency and reduce misregistration but requires technical adaptations of the PET components; additionally, both sequential and integrated PET/MRI systems require a novel method to correct for the attenuation of PET photons .
Whereas the CT component of PET/CT directly provides electron density information that can be readily used to generate attenuation-corrected PET images, the MRI signal acquired during simultaneous PET/MRI instead correlates with proton density and tissue T1/T2 properties. Current approaches to MRI-based attenuation correction (AC) include segmentation-based and atlas-based methods . Segmentation-based AC is used clinically and relies on the Dixon method to classify voxels as soft tissue (i.e., muscle and solid organs), fat, lung, or air. In contrast to the atlas-based method, which fits pre-existing averaged imaging data sets to an acquired study and is currently used mainly in the research setting, the segmentation-based method uses each patient’s own imaging data and thus can account for large tumors, postsurgical changes, anatomic variants, and other findings not readily incorporated into imaging atlases. However, segmentation-based AC has its own set of limitations. Cortical bone, which attenuates PET photons more than soft tissue, does not provide adequate signal to be represented in AC maps derived from the current clinically available MRI-based segmentation methods. Consequently, cortical bone is not accounted for by the standard Dixon method, resulting in lower standardized uptake values (SUVs) for tissues within or immediately adjacent to cortical bone when assessed by PET/MRI compared to PET/CT . Segmentation of the lung parenchyma can occasionally fail due to the relative lack of protons available to provide MR signal compared to fat or soft tissue, resulting in artifacts that propagate into the attenuation-corrected PET images and may compromise interpretation . Additionally, patient-positioning devices, such as the headphones routinely used in brain MRI, can also artifactually lower SUVs derived from MR-based AC methods . In general, compared to CT-based AC, the maximum reductions in SUV measurements derived from MR-based AC are typically on the order of 10–20%, although the diagnostic impact of this SUV underestimation on routine clinical oncologic imaging with PET/MRI appears to be relatively minor.
Workflow Optimization and Protocol Design
Given the relative novelty of PET/MRI, no standardized acquisition protocols exist. This protocol variability throughout the PET/MRI literature can make comparing the results of different studies challenging. Importantly, protocol optimization and sequence selection have been extensively described in the literature ( Table 1 ). Regardless of the acquisition details, there are basic principles that should apply to clinical protocol development: (1) PET/MRI protocols should be designed to compete with PET/CT in terms of examination duration, and (2) PET/MRI protocols should be tailored to the clinical question at hand, with the goal of creating added value beyond what PET/CT or MRI alone might otherwise provide. At our institution, initial staging studies generally include both whole-body sequences aimed at identifying distant metastases and high-resolution, anatomically focused MR sequences in the region of the primary tumor to facilitate assessment of local invasion and detection of regional metastases.
TABLE 1
Publications Addressing PET/MRI Workflow Considerations, Protocol Design, and Sequence Optimization
Fowler et al. Whole-body simultaneous positron emission tomography (PET)-MR: optimization and adaptation of MRI sequences . Von Schulthess et al. Workflow considerations in PET/MR imaging . Vargas et al. Approaches for the optimization of MR protocols in clinical hybrid PET/MRI studies . Barbosa et al. Workflow in simultaneous PET/MRI . Martinez-Möller et al. Workflow and scan protocol considerations for integrated whole-body PET/MRI in oncology . Kalemis et al. Sequential whole-body PET/MR scanner: concept, clinical use, and optimization after two years in the clinic. The manufacturer’s perspective . Reiner et al. Protocol requirements and diagnostic value of PET/MR imaging for liver metastasis detection .
Get Radiology Tree app to read full this article<
Economic Considerations
Get Radiology Tree app to read full this article<
Emerging Clinical Applications and Evidence
Get Radiology Tree app to read full this article<
Whole-body Staging
Comparing PET/MRI to PET/CT
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Radiation Reduction
Get Radiology Tree app to read full this article<
Specific Indications
Intracranial Neoplasms
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Head and Neck Neoplasms
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Thoracic Neoplasms
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Abdominal Neoplasms
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Pelvic Neoplasms
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Future Directions
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
TABLE 2
Selected Studies Demonstrating Advantages of PET/MRI Over PET/CT
Study Primary Malignancy Findings (PET/MRI Relative to PET/CT)P Value Schaarschmidt et al. Various Fewer indeterminate incidental lesions <0.001 Catalano et al. Various More clinically significant findings <0.001 Beiderwellen et al. Various Higher conspicuity of OMs <0.05 Eiber et al. Various Better anatomic delineation of OMs 0.0001 Kanda et al. Head and neck SCC Superior T staging accuracy 0.041 Grueneisen et al. Breast Superior T staging accuracy <0.05 Donati et al. Various Greater sensitivity for liver metastases 0.002 Nagamachi et al. Pancreatic Better benign vs. malignant differentiation 0.005 Queiroz et al. Gynecologic Superior T staging accuracy <0.001 Kitajima et al. Gynecologic Higher sensitivity for local recurrence 0.041 Park et al. Prostate Better identification of high-grade tumors <0.01
CT, computed tomography; MRI, magnetic resonance imaging; OMs, osseous metastases; PET, positron emission tomography; SCC, squamous cell carcinoma.
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
TABLE 3
Comparison of PET/MRI, PET/CT, and MRI in Oncologic Imaging
Potential advantages of PET/MRI over PET/CT
Potential advantages of PET/MRI over MRI
Potential advantages of PET/CT over PET/MRI
CT, computed tomography; FDG, 2-deoxy-2-[ 18 F]fluoro-D-glucose; MRI, magnetic resonance imaging; PET, positron emission tomography.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Kostakoglu L., Agress H., Goldsmith S.J.: Clinical role of FDG PET in evaluation of cancer patients. Radiographics 2003; 23: pp. 315-340.
2. Fletcher J.W., Djulbegovic B., Soares H.P., et. al.: Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med 2008; 49: pp. 480-508.
3. Ben-Haim S., Ell P.: 18 F-FDG PET and PET/CT in the evaluation of cancer treatment response. J Nucl Med 2009; 50: pp. 88-99.
4. Treglia G., Sadeghi R., Del Sole A., et. al.: Diagnostic performance of PET/CT with tracers other than F-18-FDG in oncology: an evidence-based review. Clin Transl Oncol 2014; 16: pp. 770-775.
5. Kalemis A., Delattre B.M.A., Heinzer S.: Sequential whole-body PET/MR scanner: concept, clinical use, and optimisation after two years in the clinic. The manufacturer’s perspective. MAGMA 2013; 26: pp. 5-23.
6. Hofmann M., Bezrukov I., Mantlik F., et. al.: MRI-based attenuation correction for whole-body PET/MRI: quantitative evaluation of segmentation- and atlas-based methods. J Nucl Med 2011; 52: pp. 1392-1399.
7. Hofmann M., Steinke F., Scheel V., et. al.: MRI-based attenuation correction for PET/MRI: a novel approach combining pattern recognition and atlas registration. J Nucl Med 2008; 49: pp. 1875-1883.
8. Aznar M.C., Sersar R., Saabye J., et. al.: Whole-body PET/MRI: the effect of bone attenuation during MR-based attenuation correction in oncology imaging. Eur J Radiol 2014; 83: pp. 1177-1183.
9. Keller S.H., Holm S., Hansen A.E., et. al.: Image artifacts from MR-based attenuation correction in clinical, whole-body PET/MRI. MAGMA 2013; 26: pp. 173-181.
10. Ferguson A., McConathy J., Su Y., et. al.: Attenuation effects of MR headphones during brain PET/MR studies. J Nucl Med Technol 2014; 42: pp. 93-100.
11. Fowler K.J., McConathy J., Narra V.R.: Whole-body simultaneous positron emission tomography (PET)-MR: optimization and adaptation of MRI sequences. J Magn Reson Imaging 2014; 39: pp. 259-268.
12. Von Schulthess G.K., Veit-Haibach P.: Workflow considerations in PET/MR imaging. J Nucl Med 2014; 55: pp. 19S-24S.
13. Vargas M.-I., Becker M., Garibotto V., et. al.: Approaches for the optimization of MR protocols in clinical hybrid PET/MRI studies. MAGMA 2013; 26: pp. 57-69.
14. Barbosa F.G., von Schulthess G., Veit-Haibach P.: Workflow in simultaneous PET/MRI. Semin Nucl Med 2015; 45: pp. 332-344.
15. Martinez-Möller A., Eiber M., Nekolla S.G., et. al.: Workflow and scan protocol considerations for integrated whole-body PET/MRI in oncology. J Nucl Med 2012; 53: pp. 1415-1426.
16. Reiner C.S., Stolzmann P., Husmann L., et. al.: Protocol requirements and diagnostic value of PET/MR imaging for liver metastasis detection. Eur J Nucl Med Mol Imaging 2014; 41: pp. 649-658.
17. Drzezga A., Souvatzoglou M., Eiber M., et. al.: First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med 2012; 53: pp. 845-855.
18. Heusch P., Nensa F., Schaarschmidt B., et. al.: Diagnostic accuracy of whole-body PET/MRI and whole-body PET/CT for TNM staging in oncology. Eur J Nucl Med Mol Imaging 2015; 42: pp. 42-48.
19. Tian J., Fu L., Yin D., et. al.: Does the novel integrated PET/MRI offer the same diagnostic performance as PET/CT for oncological indications?. PLoS ONE 2014; 9: pp. e90844.
20. Van Ufford H.M.E.Q., Kwee T.C., Beek F.J., et. al.: Newly diagnosed lymphoma: initial results with whole-body T1-weighted, STIR, and diffusion-weighted MRI compared with 18 F-FDG PET/CT. AJR Am J Roentgenol 2011; 196: pp. 662-669.
21. Donati O.F., Hany T.F., Reiner C.S., et. al.: Value of retrospective fusion of PET and MR images in detection of hepatic metastases: comparison with 18F-FDG PET/CT and Gd-EOB-DTPA-enhanced MRI. J Nucl Med 2010; 51: pp. 692-699.
22. Buchbender C., Hartung-Knemeyer V., Beiderwellen K., et. al.: Diffusion-weighted imaging as part of hybrid PET/MRI protocols for whole-body cancer staging: does it benefit lesion detection?. Eur J Radiol 2013; 82: pp. 877-882.
23. Heusch P., Buchbender C., Beiderwellen K., et. al.: Standardized uptake values for [ 18 F] FDG in normal organ tissues: comparison of whole-body PET/CT and PET/MRI. Eur J Radiol 2013; 82: pp. 870-876.
24. Iagaru A., Mittra E., Minamimoto R.: Simultaneous whole-body time-of-flight 18 F-FDG PET/MRI: a pilot study comparing SUVmax with PET/CT and assessment of MR image quality. Clin Nucl Med 2015; 40: pp. 1-8.
25. Beiderwellen K., Huebner M., Heusch P., et. al.: Whole-body [ 18 F]FDG PET/MRI vs. PET/CT in the assessment of bone lesions in oncological patients: initial results. Eur Radiol 2014; 24: pp. 2023-2030.
26. Eiber M., Takei T., Souvatzoglou M., et. al.: Performance of whole-body integrated 18 F-FDG PET/MR in comparison to PET/CT for evaluation of malignant bone lesions. J Nucl Med 2014; 55: pp. 191-197.
27. Huang B., Law M.W.-M., Khong P.-L.: Whole-body PET/CT scanning: estimation of radiation dose and cancer risk. Radiology 2009; 251: pp. 166-174.
28. Schaarschmidt B.M., Grueneisen J., Heusch P., et. al.: Does 18 F-FDG PET/MRI reduce the number of indeterminate abdominal incidentalomas compared with 18 F-FDG PET/CT?. Nucl Med Commun 2015; 36: pp. 588-595.
29. Catalano O.A., Rosen B.R., Sahani D.V., et. al.: Clinical impact of PET/MR imaging in patients with cancer undergoing same-day PET/CT: initial experience in 134 patients—a hypothesis-generating exploratory study. Radiology 2013; 269: pp. 857-869.
30. Ledezma C.J., Chen W., Sai V., et. al.: 18 F-FDOPA PET/MRI fusion in patients with primary/recurrent gliomas: initial experience. Eur J Radiol 2009; 71: pp. 242-248.
31. Navarria P., Reggiori G., Pessina F., et. al.: Investigation on the role of integrated PET/MRI for target volume definition and radiotherapy planning in patients with high grade glioma. Radiother Oncol 2014; 112: pp. 425-429.
32. Berntsson S.G., Falk A., Savitcheva I., et. al.: Perfusion and diffusion MRI combined with 11 C-methionine PET in the preoperative evaluation of suspected adult low-grade gliomas. J Neurooncol 2013; 114: pp. 241-249.
33. Filss C.P., Galldiks N., Stoffels G., et. al.: Comparison of 18 F-FET PET and perfusion-weighted MR imaging: a PET/MR imaging hybrid study in patients with brain tumors. J Nucl Med 2014; 55: pp. 540-545.
34. Spence A.M., Muzi M., Swanson K.R., et. al.: Regional hypoxia in glioblastoma multiforme quantified with [ 18 F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clin Cancer Res 2008; 14: pp. 2623-2630.
35. Vaupel P., Mayer A.: Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev 2007; 26: pp. 225-239.
36. Yoon J.H., Kim J., Kang W.J., et. al.: Grading of cerebral glioma with multiparametric MR imaging and 18 F-FDG-PET: concordance and accuracy. Eur Radiol 2014; 24: pp. 380-389.
37. Gempt J., Soehngen E., Förster S., et. al.: Multimodal imaging in cerebral gliomas and its neuropathological correlation. Eur J Radiol 2014; 83: pp. 829-834.
38. Schwarzenberg J., Czernin J., Cloughesy T.F., et. al.: Treatment response evaluation using 18 F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res 2014; 20: pp. 3550-3559.
39. Tanaka Y., Nariai T., Momose T., et. al.: Glioma surgery using a multimodal navigation system with integrated metabolic images. J Neurosurg 2009; 110: pp. 163-172.
40. Momose T., Nariai T., Kawabe T., et. al.: Clinical benefit of 11 C methionine PET imaging as a planning modality for radiosurgery of previously irradiated recurrent brain metastases. Clin Nucl Med 2014; 39: pp. 939-943.
41. Terakawa Y., Tsuyuguchi N., Iwai Y., et. al.: Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 2008; 49: pp. 694-699.
42. Becker M., Zaidi H.: Imaging in head and neck squamous cell carcinoma: the potential role of PET/MRI. Br J Radiol 2014; 87: pp. 20130677.
43. Kanda T., Kitajima K., Suenaga Y., et. al.: Value of retrospective image fusion of 18 F-FDG PET and MRI for preoperative staging of head and neck cancer: comparison with PET/CT and contrast-enhanced neck MRI. Eur J Radiol 2013; 82: pp. 2005-2010.
44. Queiroz M.A., Hüllner M., Kuhn F., et. al.: Use of diffusion-weighted imaging (DWI) in PET/MRI for head and neck cancer evaluation. Eur J Nucl Med Mol Imaging 2014; 41: pp. 2212-2221.
45. Queiroz M.A., Hüllner M., Kuhn F., et. al.: PET/MRI and PET/CT in follow-up of head and neck cancer patients. Eur J Nucl Med Mol Imaging 2014; 41: pp. 1066-1075.
46. Partovi S., Kohan A., Vercher-Conejero J.L., et. al.: Qualitative and quantitative performance of 18 F-FDG-PET/MRI versus 18 F-FDG-PET/CT in patients with head and neck cancer. AJNR Am J Neuroradiol 2014; 35: pp. 1970-1975.
47. Varoquaux A., Rager O., Poncet A., et. al.: Detection and quantification of focal uptake in head and neck tumours: (18) F-FDG PET/MR versus PET/CT. Eur J Nucl Med Mol Imaging 2014; 41: pp. 462-475.
48. Rauscher I., Eiber M., Fürst S., et. al.: PET/MR imaging in the detection and characterization of pulmonary lesions: technical and diagnostic evaluation in comparison to PET/CT. J Nucl Med 2014; 55: pp. 724-729.
49. Schwenzer N., Schraml C., Müller M.: Pulmonary lesion assessment: comparison of whole-body hybrid MR/PET and PET/CT imaging—pilot study. Radiology 2012; 264: pp. 551-558.
50. Heusch P., Buchbender C., Köhler J., et. al.: Thoracic staging in lung cancer: prospective comparison of 18 F-FDG PET/MR imaging and 18 F-FDG PET/CT. J Nucl Med 2014; 55: pp. 373-378.
51. Plathow C., Aschoff P., Lichy M.P., et. al.: Positron emission tomography/computed tomography and whole-body magnetic resonance imaging in staging of advanced nonsmall cell lung cancer—initial results. Invest Radiol 2008; 43: pp. 290-297.
52. Ohno Y., Koyama H., Yoshikawa T., et. al.: N stage disease in patients with non-small cell lung cancer: efficacy of quantitative and qualitative assessment with STIR turbo spin-echo imaging, and fluorodeoxyglucose PET/CT. Radiology 2011; 261: pp. 605-615.
53. Kohan A., Kolthammer J., Vercher-Conejero J., et. al.: N staging of lung cancer patients with PET/MRI using a three-segment model attenuation correction algorithm: initial experience. Eur Radiol 2013; 23: pp. 3161-3169.
54. Lee K.H., Park C.M., Lee S.M., et. al.: Pulmonary nodule detection in patients with a primary malignancy using hybrid PET/MRI: is there value in adding contrast-enhanced MR imaging?. PLoS ONE 2015; 10: pp. e0129660.
55. Pace L., Nicolai E., Luongo A., et. al.: Comparison of whole-body PET/CT and PET/MRI in breast cancer patients: lesion detection and quantitation of 18 F-deoxyglucose uptake in lesions and in normal organ tissues. Eur J Radiol 2014; 83: pp. 289-296.
56. Grueneisen J., Nagarajah J., Buchbender C., et. al.: Positron emission tomography/magnetic resonance imaging for local tumor staging in patients with primary breast cancer: a comparison with positron emission tomography/computed tomography and magnetic resonance imaging. Invest Radiol 2015; 50: pp. 505-513.
57. Lee G., I H., Kim S.-J., et. al.: Clinical implication of PET/MR imaging in preoperative esophageal cancer staging: comparison with PET/CT, endoscopic ultrasonography, and CT. J Nucl Med 2014; 55: pp. 1242-1247.
58. Fosse P., Girault S., Hoareau J., et. al.: Unusual uptake of 18 FDG by a hepatic adenoma. Clin Nucl Med 2013; 38: pp. 135-136.
59. Beiderwellen K., Gomez B., Buchbender C., et. al.: Depiction and characterization of liver lesions in whole body [ 18 F]-FDG PET/MRI. Eur J Radiol 2013; 82: pp. e669-e675.
60. Nagamachi S., Nishii R., Wakamatsu H., et. al.: The usefulness of (18) F-FDG PET/MRI fusion image in diagnosing pancreatic tumor: comparison with (18)F-FDG PET/CT. Ann Nucl Med 2013; 27: pp. 554-563.
61. Hofmann M., Maecke H., Börner R., et. al.: Biokinetics and imaging with the somatostatin receptor PET radioligand (68) Ga-DOTATOC: preliminary data. Eur J Nucl Med 2001; 28: pp. 1751-1757.
62. Mayerhoefer M.E., Ba-Ssalamah A., Weber M., et. al.: Gadoxetate-enhanced versus diffusion-weighted MRI for fused Ga-68-DOTANOC PET/MRI in patients with neuroendocrine tumours of the upper abdomen. Eur Radiol 2013; 23: pp. 1978-1985.
63. Gaertner F., Beer A., Souvatzoglou M., et. al.: Evaluation of feasibility and image quality of 68 Ga-DOTATOC positron emission tomography/magnetic resonance in comparison with positron emission tomography/computed tomography in patients with neuroendocrine tumors. Invest Radiol 2013; 48: pp. 263-272.
64. Beiderwellen K.J., Poeppel T.D., Hartung-Knemeyer V., et. al.: Simultaneous 68Ga-DOTATOC PET/MRI in patients with gastroenteropancreatic neuroendocrine tumors: initial results. Invest Radiol 2013; 48: pp. 273-279.
65. Hope T.A., Pampaloni M.H., Nakakura E., et. al.: Simultaneous (68) Ga-DOTA-TOC PET/MRI with gadoxetate disodium in patients with neuroendocrine tumor. Abdom Imaging 2015; 40: pp. 1432-1440.
66. Blanchet E., Millo C., Martucci V.: Integrated whole-body PET/MRI with 18 F-FDG, 18 F-FDOPA, and 18 F-FDA in paragangliomas in comparison with PET/CT. Clin Nucl Med 2013; 39: pp. 243-250.
67. Kitajima K., Suenaga Y., Ueno Y., et. al.: Fusion of PET and MRI for staging of uterine cervical cancer: comparison with contrast-enhanced (18) F-FDG PET/CT and pelvic MRI. Clin Imaging 2014; 38: pp. 464-469.
68. Kitajima K., Suenaga Y., Ueno Y., et. al.: Value of fusion of PET and MRI for staging of endometrial cancer: comparison with 18 F-FDG contrast-enhanced PET/CT and dynamic contrast-enhanced pelvic MRI. Eur J Radiol 2013; 82: pp. 1672-1676.
69. Queiroz M.A., Kubik-Huch R.A., Hauser N., et. al.: PET/MRI and PET/CT in advanced gynaecological tumours: initial experience and comparison. Eur Radiol 2015; 25: pp. 2222-2230.
70. Kitajima K., Suenaga Y., Ueno Y., et. al.: Value of fusion of PET and MRI in the detection of intra-pelvic recurrence of gynecological tumor: comparison with 18 F-FDG contrast-enhanced PET/CT and pelvic MRI. Ann Nucl Med 2014; 28: pp. 25-32.
71. Beiderwellen K., Grueneisen J., Ruhlmann V., et. al.: [ (18) F]FDG PET/MRI vs. PET/CT for whole-body staging in patients with recurrent malignancies of the female pelvis: initial results. Eur J Nucl Med Mol Imaging 2015; 42: pp. 56-65.
72. Grueneisen J., Beiderwellen K., Heusch P., et. al.: Simultaneous positron emission tomography/magnetic resonance imaging for whole-body staging in patients with recurrent gynecological malignancies of the pelvis: a comparison to whole-body magnetic resonance imaging alone. Invest Radiol 2014; 49: pp. 808-815.
73. Grueneisen J., Beiderwellen K., Heusch P., et. al.: Correlation of standardized uptake value and apparent diffusion coefficient in integrated whole-body PET/MRI of primary and recurrent cervical cancer. PLoS ONE 2014; 9: pp. e96751.
74. Grueneisen J., Schaarschmidt B.M., Beiderwellen K., et. al.: Diagnostic value of diffusion-weighted imaging in simultaneous 18 F-FDG PET/MR imaging for whole-body staging of women with pelvic malignancies. J Nucl Med 2014; 55: pp. 1930-1935.
75. Schoots I.G., Roobol M.J., Nieboer D., et. al.: Magnetic resonance imaging–targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 2015; 68: pp. 438-450.
76. Röthke M., Blondin D., Schlemmer H.-P., et. al.: [PI-RADS classification: structured reporting for MRI of the prostate]. Rofo 2013; 185: pp. 253-261.
77. Jadvar H.: Molecular imaging of prostate cancer with PET. J Nucl Med 2013; 54: pp. 1685-1688.
78. Park H., Wood D., Hussain H., et. al.: Introducing parametric fusion PET/MRI of primary prostate cancer. J Nucl Med 2012; 53: pp. 546-551.
79. Kim Y.-I., Cheon G.J., Paeng J.C., et. al.: Usefulness of MRI-assisted metabolic volumetric parameters provided by simultaneous (18) F-fluorocholine PET/MRI for primary prostate cancer characterization. Eur J Nucl Med Mol Imaging 2015; 42: pp. 1247-1256.
80. Hartenbach M., Hartenbach S., Bechtloff W., et. al.: Combined PET/MRI improves diagnostic accuracy in patients with prostate cancer: a prospective diagnostic trial. Clin Cancer Res 2014; 20: pp. 3244-3253.
81. Jambor I., Borra R., Kemppainen J., et. al.: Improved detection of localized prostate cancer using co-registered MRI and 11 C-acetate PET/CT. Eur J Radiol 2012; 81: pp. 2966-2972.
82. Afshar-Oromieh A., Haberkorn U., Schlemmer H., et. al.: Comparison of PET/CT and PET/MRI hybrid systems using a 68 Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: initial experience. Eur J Nucl Med Mol Imaging 2014; 41: pp. 887-897.
83. Piccardo A., Paparo F., Picazzo R.: Value of fused 18 F-choline-PET/MRI to evaluate prostate cancer relapse in patients showing biochemical recurrence after EBRT: preliminary results. BioMed Res Int 2014; 2014: 103718
84. Paspulati R.M., Partovi S., Herrmann K.A., et. al.: Comparison of hybrid FDG PET/MRI compared with PET/CT in colorectal cancer staging and restaging: a pilot study. Abdom Imaging 2015; 40: pp. 1415-1425.
85. Furst S., Grimm R., Hong I., et. al.: Motion correction strategies for integrated PET/MR. J Nucl Med 2015; 56: pp. 261-269.
![Figure 1, 29-year-old woman with newly diagnosed cervical cancer presented for initial staging. Coronal T2-weighted images (T2WIs) with 2-deoxy-2-[ 18 F]fluoro-D-glucose-positron emission tomography (FDG-PET) fusion ( a posterior to b ) revealed marked tracer uptake by an infiltrative mass ( arrow ) just inferior to the uterus ( arrowhead ) and posterior to the urinary bladder ( asterisk ), compatible with the patient's known cervical cancer. There was no evidence of pelvic nodal disease or distant metastases. This case highlights the potential of PET/magnetic resonance imaging (MRI) to serve as a whole-body imaging modality for multiple oncologic indications. (Color version of figure available online.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/PETMRI/0_1s20S1076633215004171.jpg)
![Figure 2, 69-year-old man with known metastatic papillary thyroid carcinoma presented for restaging. Sagittal computed tomography (CT) images (a) revealed a subtle lytic lesion within the T2 vertebral body ( arrow head ) but a normal appearance of the T5 vertebral body ( arrow ). Sagittal CT images with 2-deoxy-2-[ 18 F]fluoro-D-glucose-positron emission tomography (FDG-PET) fusion (b) demonstrated an FDG-avid focus in the T2 ( arrowhead ) vertebral body highly suspicious for metastatic disease. In contrast, a focus of more subtly increased FDG uptake in the T5 vertebral body ( arrow ) without a CT correlate was felt to be indeterminate, as both metastatic disease and degenerative disease could conceivably produce this appearance. Sagittal T1-weighted images (T1WIs) (c) from subsequent PET/magnetic resonance imaging (MRI) showed clear evidence of marrow replacement in the T2 ( arrowhead ) and T5 ( arrow ) vertebral bodies. Corresponding foci of FDG avidity on sagittal T1WIs with FDG-PET fusion (d) strongly supported the presence of metastases at both sites. These images show an example of the improved anatomic delineation of malignant osseous disease with PET/MRI relative to PET/CT, as well as the power of PET/MRI to distinguish osseous malignancy from degenerative remodeling. (Color version of figure available online.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/PETMRI/1_1s20S1076633215004171.jpg)
![Figure 3, 8-year-old girl with suspected recurrent small cell glioma presented for restaging. (a) Transaxial contrast-enhanced T1-weighted images (T1WIs) showed a heterogeneous mass ( arrows ) centered in the right basal ganglia. Multiple small nonenhancing areas ( asterisks ) interspersed among numerous enhancing foci were noted. (b) Transaxial contrast-enhanced T1WIs with 6-[ 18 F]fluoro-3,4-dihydroxy-phenylalanine-positron emission tomography (FDOPA-PET) fusion revealed avid tracer uptake by the enhancing and nonenhancing portions of this mass ( arrows ), illustrating transport of the FDOPA tracer into areas of tumor involvement where the blood-brain barrier was still intact. (c) Transaxial T2-weighted images (T2WIs) acquired with a fluid-attenuated inversion recovery (FLAIR) sequence demonstrated abnormal T2 prolongation within the entire area of increased FDOPA uptake ( arrows ), further supporting tumor infiltration into these regions. Tumor invasion into the right temporal lobe ( asterisks ) was also suspected, although without a corresponding focus of FDOPA uptake. This case highlights one of the advantages of PET/magnetic resonance imaging (MRI) relative to MRI alone in the setting of neuro-oncology, as certain tracers can enter the central nervous system to delineate tumor involvement in anatomic regions where the blood-brain barrier remains intact. (Color version of figure available online.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/PETMRI/2_1s20S1076633215004171.jpg)
![Figure 4, 64-year-old woman with remote history of facial nerve sparing left parotidectomy for adenoid cystic carcinoma presented with new onset of left facial nerve paralysis. Transaxial T1-weighted images (T1WIs) with 2-deoxy-2-[ 18 F]fluoro-D-glucose-positron emission tomography (FDG-PET) fusion revealed (a) a hypermetabolic mass ( asterisk ) involving the superficial and deep left parotid spaces, (b) increased FDG uptake in the region of the left mental foramen ( arrowhead ), and (d) a focus of FDG avidity at the left mandibular foramen. (c) Transaxial T1WIs demonstrated subtle enlargement of the left mandibular foramen ( arrow ) compared to the contralateral side ( not shown ). These findings were consistent with recurrent malignancy and perineural spread along the expected course of the left inferior alveolar nerve, a diagnosis that likely would have been challenging with PET/computed tomography (CT) or magnetic resonance imaging (MRI) alone. In this regard, PET/MRI can facilitate accurate T staging of head and neck carcinoma. (Color version of figure available online.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/PETMRI/3_1s20S1076633215004171.jpg)
![Figure 5, 60-year-old woman with a remote history of treated cervical cancer was referred from an outside institution for evaluation of a lung mass. (a) Transaxial contrast-enhanced T1-weighted images (T1WIs) revealed a spiculated right parahilar lung mass ( asterisk ) with mediastinal invasion and near-encasement of the superior vena cava ( arrow ). (b) Transaxial contrast-enhanced T1WIs with 2-deoxy-2-[ 18 F]fluoro-D-glucose-positron emission tomography (FDG-PET) fusion at this same level showed the lesion ( asterisk ) to be hypermetabolic and also identified metastatic lesions of the thoracic spine ( arrowhead ) and left lung hilum ( arrow ). (c) Transaxial contrast-enhanced T1WIs obtained at a more superior level demonstrated lateral extension of the right parahilar lung mass ( asterisk ) with invasion of the chest wall ( arrow ). (d) Transaxial contrast-enhanced T1WIs with FDG-PET fusion at this same level revealed additional sites of metastatic disease in the right ( arrowhead ) and left ( arrow ) aspects of the mediastinum. Subsequent computed tomography (CT)-guided biopsy was positive for squamous cell carcinoma. Given the imaging appearance of the right parahilar mass and the distribution of FDG-avid lymph nodes, these findings were favored to represent metastatic bronchogenic carcinoma rather than recurrence of the patient's treated cervical cancer. These images demonstrate the utility of PET/magnetic resonance imaging (MRI) in detecting and characterizing mediastinal and chest wall invasion by intrathoracic malignancies. (Color version of figure available online.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/PETMRI/4_1s20S1076633215004171.jpg)
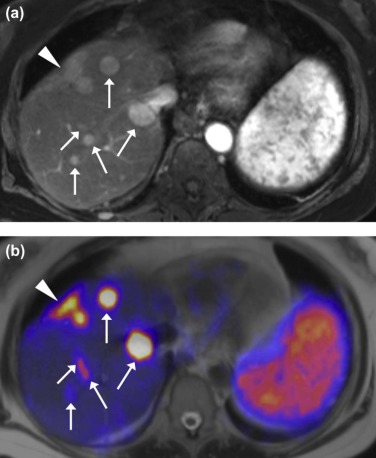
![Figure 6, 70-year-old man with cecal adenocarcinoma status post right hemicolectomy presented for restaging. (a) Transaxial computed tomography (CT) images of the liver revealed small hypodense lesions ( arrows ) that were deemed too small to characterize. Positron emission tomography/magnetic resonance imaging (PET/MRI) was subsequently performed for further evaluation. (b) Transaxial contrast-enhanced T1-weighted images (T1WIs) obtained in the hepatocellular phase of contrast demonstrated two hypoenhancing foci ( arrows ) near the liver dome. (c) Transaxial contrast-enhanced T1WIs with 2-deoxy-2-[ 18 F]fluoro-D-glucose (FDG)-PET fusion showed no definite FDG-avid correlate for these lesions ( arrows ), likely because of their small size and relatively low FDG uptake compared to normal liver. (d) However, diffusion-weighted imaging (DWI) revealed marked diffusion restriction within these lesions ( arrows ) compatible with hypercellular hepatic metastases. This case demonstrates how PET/MRI, when incorporating DWI of the liver, can increase the conspicuity of (and likely also the sensitivity for) hepatic metastases, relative to PET/CT. (Color version of figure available online.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/PETMRI/5_1s20S1076633215004171.jpg)
![Figure 8, 72-year-old woman with a new diagnosis of vaginal small cell carcinoma presented for initial staging. (a) Transaxially-acquired nonisotropic T2-weighted images (T2WIs) showed a tumor ( asterisk ) centered along the anterior aspect of the vaginal canal (v), with extension anteriorly toward the urinary bladder (bl) and posteriorly toward the rectum (r). (b) Sagittal reformat of these nonisotropic T2WIs resulted in a significant degradation of the image quality. Assessment of the relationship between the tumor ( asterisk ) and the adjacent structures was difficult. Transaxially-acquired isotropic T2WIs (c) with 2-deoxy-2-[ 18 F]fluoro-D-glucose-positron emission tomography (FDG-PET) fusion (e) showed invasion of the tumor ( asterisk ) into the posterior urinary bladder wall ( arrow ) and the anterior rectal wall ( arrowhead ). Sagittal reformat of these isotropic T2WIs (d) with FDG-PET fusion (f) much more clearly depicted the full craniocaudal extent of tumor ( asterisk ) invasion into the posterior urinary bladder wall ( arrow ). This case demonstrates the advantages of isotropic magnetic resonance (MR) sequences for the accurate and confident local staging of primary tumors via PET/MRI. (Color version of figure available online.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/PETMRI/7_1s20S1076633215004171.jpg)
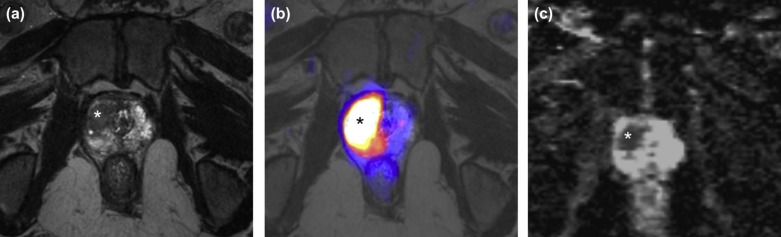
![Figure 9, 46-year old woman with newly diagnosed squamous cell carcinoma of the cervix presented for initial staging. Transaxial computed tomography (CT) images (a) with 2-deoxy-2-[ 18 F]fluoro-D-glucose-positron emission tomography (FDG-PET) fusion (c) demonstrated a hypermetabolic soft-tissue nodule ( arrow ) in the region of the right external iliac artery and similar focus of less-intense uptake near the left external iliac artery ( arrowhead ). Differential considerations included nodal metastases, left ovarian metastases, and physiological ovarian uptake. PET/magnetic resonance imaging (MRI) was subsequently performed for further evaluation. Transaxial high-resolution T2-weighted images (T2WIs) (b) with FDG-PET fusion (d) revealed ovoid structures with internal T2-hyperintense cystic spaces compatible with ovaries. There was significant FDG uptake on the right ( arrow ) and trace FDG uptake on the ( left ), similar to the PET findings from the PET/CT examination. Consequently, nodal metastases were excluded from the differential. As with the prior case, the superior soft-tissue contrast of PET/MRI relative to PET/CT also promotes accurate N staging of gynecologic malignancies. (Color version of figure available online.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/PETMRI/8_1s20S1076633215004171.jpg)
![Figure 11, 50-year-old woman with newly diagnosed rectal adenocarcinoma presented for initial staging. Transaxial isotropic T2-weighted images (T2WIs) (a) with 2-deoxy-2-[ 18 F]fluoro-D-glucose-positron emission tomography (FDG-PET) fusion (b) showed effacement of the fat plane ( long arrow ) between the rectum (r) and the vagina (v) by a large, hypermetabolic tumor arising from the rectum. The fat plane between the urinary bladder (bl) and the vagina was preserved, indicating bladder sparing. Coronal reformats of these isotropic T2WIs (c) with FDG-PET fusion (d) revealed a 10 × 8 mm right internal iliac lymph node ( arrowhead ) that failed to meet size criteria for lymphadenopathy but displayed FDG avidity highly suspicious for nodal metastatic disease. These images demonstrate the ability of PET/magnetic resonance imaging (MRI) to detect tumor spread to lymph nodes that might not appear suspicious on MRI alone because of their normal size and/or morphology, potentially resulting in clinically significant upstaging. (Color version of figure available online.)](https://storage.googleapis.com/dl.dentistrykey.com/clinical/PETMRI/10_1s20S1076633215004171.jpg)