Rationale and Objectives
The aim of this study was to assess copper metabolism of human hepatocellular carcinoma (HCC) with positron emission tomographic (PET) imaging using copper (II)-64 chloride ( 64 CuCl 2 ) as a tracer.
Materials and Methods
PET imaging of athymic mice ( n = 5) bearing extrahepatic HCC xenografts was performed 24 hours after the intravenous injection of 64 CuCl 2 , followed by ex vivo tissue radioactivity assay. Expression of human copper transporter 1 (hCTR1) in HCC cells and tissues was examined by real-time reverse transcription polymerase chain reaction and immunohistochemistry analysis, respectively.
Results
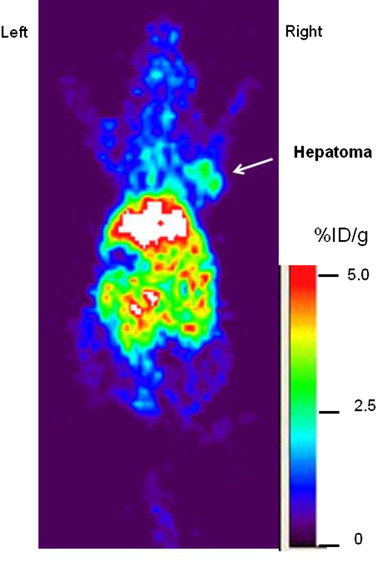
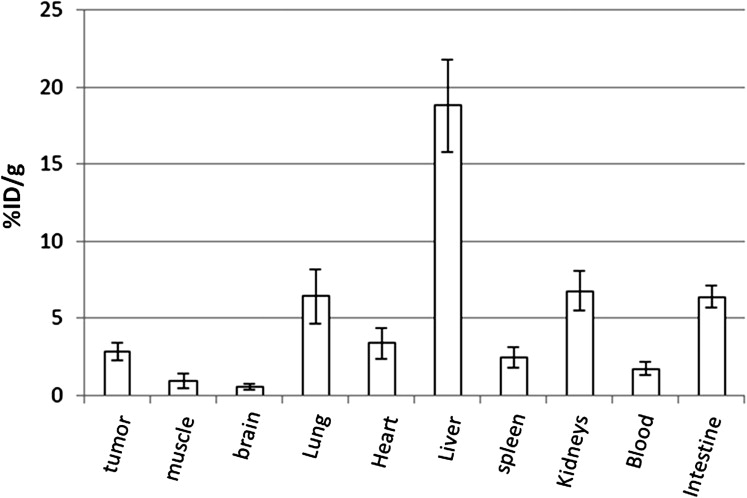
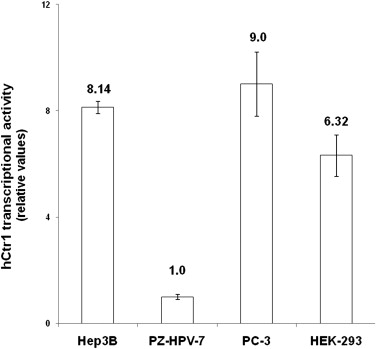
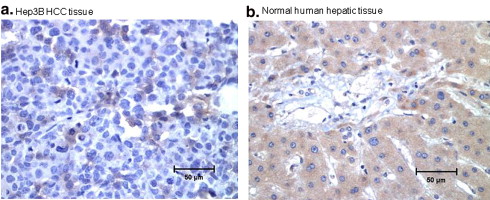
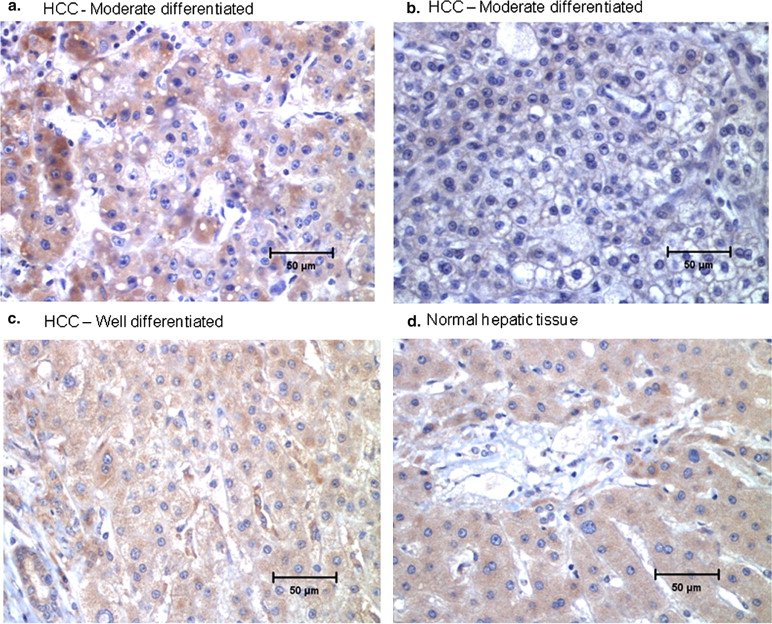
The extrahepatic HCC xenografts in mice with increased uptake of 64 Cu radionuclide were visualized on the micro-PET images obtained 24 hours after the intravenous injection of 64 CuCl 2 . PET quantitative analysis revealed increased 64 Cu radioactivity in tumor tissues (2.7 ± 0.6 %ID/g) compared to that in the soft tissue of the left shoulder opposite to the tumor site (0.6 ± 0.2 %ID/g) and the brain (0.7 ± 0.1 %ID/g) but lower than that of the liver (16.6 ± 1.3 %ID/g). Expression of hCTR1 in the HCC cells and xenograft tumor tissues was demonstrated by real-time reverse transcription polymerase chain reaction and immunohistochemistry analysis, respectively. The expression level of hCTR1 in the Hep3B HCC xenograft tissues was lower than that detected in the normal hepatic tissues and the tissue samples of well-differentiated primary HCC. Variable expression of hCTR1 was detected in the tissue samples of moderately differentiated primary HCC.
Conclusions
Extrahepatic human HCC xenografts in mice could be localized with 64 CuCl 2 PET imaging, which might be useful for the localization and quantitative assessment of copper metabolism in extrahepatic metastases of HCC in humans.
Hepatocellular carcinoma (HCC) is a threat to the health of the global population, particularly to people living in Asia and sub-Saharan Africa . Significant advances have been made in the treatment of patients diagnosed with the early stages of HCC, but the prognosis for patients with extrahepatic HCC metastases is poor, because they are often resistant to systemic chemotherapy . Moreover, the prognosis of patients with intracranial metastases of HCC is dismal . Liver transplantation is a potentially curative treatment for those patients with HCC localized within the liver . In the pretransplantation workup, however, it is essential to exclude extrahepatic metastases in those patients who are considered candidates for liver transplantation. Positron emission tomographic (PET) imaging using 2-deoxy-2-[ 18 F]-fluoro- d -glucose ( 18 F-FDG) is clinically well accepted for the staging of many human cancers and has been found to be highly sensitive for the detection of the extrahepatic spread of hypermetabolic HCC . However, 18 F-FDG PET imaging is limited for the detection of intracranial HCC metastases because of the high background of FDG uptake by the normal brain tissue. Thus, it is necessary to establish an alternative tracer that may be used for the evaluation of intracranial metastases of HCC under circumstances in which the use of 18 F FDG PET imaging is limited.
Copper is an essential nutrient in mammals, and intracellular copper homeostasis in humans is regulated by a delicate network of copper transporters , which include human copper transporter 1 (hCTR1), ATP7A, ATP7B, as well as copper chaperons antioxidant protein 1, cytochrome c oxidase 17, and copper chaperone for superoxide dismutase . Copper is required for cell proliferation and tumor growth, and high concentrations of copper have been observed in many of human tumor tissues . Extrahepatic mouse hepatoma grafts and human prostate xenografts in mice could be localized with PET imaging using 64 CuCl 2 as a tracer . In this study, PET imaging of athymic mice bearing human HCC xenografts was conducted to determine whether extrahepatic human HCC metastases could be localized with 64 CuCl 2 PET imaging. To study the molecular mechanism of copper hypermetabolism in HCC, expression of hCTR1 in HCC cells and tumor tissues was examined by quantitative real-time reverse transcription polymerase chain reaction (RC-PCR) and immunohistochemistry analysis, respectively.
Materials and methods
Cells and Animal Model
Get Radiology Tree app to read full this article<
PET Imaging
Get Radiology Tree app to read full this article<
PET Quantitative Analyses of Tracer Concentration
Get Radiology Tree app to read full this article<
Radioactivity Assay of Tissue Radiotracer Concentration
Get Radiology Tree app to read full this article<
Quantitative Real-time RT-PCR
Get Radiology Tree app to read full this article<
IHC Analysis
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Visualization of Hep3B HCC Xenografts in Mice with 64 CuCl 2 PET Imaging
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Biodistribution of 64 Cu Radioactivity by PET Quantitative Analysis
Get Radiology Tree app to read full this article<
Biodistribution of 64 Cu Radioactivity by Ex Vivo Tissue Radioactivity Assay
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
High-level Transcription of hCTR1 in Hep3B Cells by Real-time RT-PCR
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Detection of hCTR1 Expression in Hep3B and Primary HCC Tissues by IHC Analysis
Get Radiology Tree app to read full this article<
Table 1
Expression of hCTR1 in Malignant and Nonmalignant Hepatic Tissues by IHC Analysis
Subject Histopathologic Diagnosis hCTR1 Immunoreactivity by IHC ∗ 1 HCC, well differentiated +++ 2 HCC, well differentiated +++ 3 HCC, moderately differentiated +++ 4 HCC, moderately differentiated +++ 5 HCC, moderately differentiated +++ 6 HCC, moderately differentiated + 7 HCC, moderately differentiated + 8 HCC, moderately differentiated ++ 9 HCC, moderately differentiated + 10 Normal liver parenchyma +++ 11 Normal liver parenchyma +++ 12 Mucinous cystadenoma with steatosis ++ 13 Benign liver hemangioma with mild portal inflammation ++ 14 Cirrhotic liver with steatosis and portal inflammation + 15 Benign liver parenchyma ++
HCC, hepatocellular carcinoma; hCTR1, human copper transporter 1; IHC, immunohistochemistry.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Acknowledgments
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Parkin D.M., Pisani P., Ferlay J.: Global cancer statistics. CA Cancer J Clin 1999; 49: pp. 33-64.
2. Pisani P., Parkin D.M., Bray F.J., et. al.: Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 1999; 83: pp. 18-29.
3. Lee Y.T., Geer A.D.: Primary liver cancer: pattern of metastasis. J Surg Oncol 1987; 36: pp. 26-31.
4. Stuart K.E., Anand A.J., Jenkins R.L.: Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer 1996; 77: pp. 2217-2222.
5. Yen F.S., Wu J.C., Lai C.R., et. al.: Clinical and radiological pictures of hepatocellular carcinoma with intracranial metastasis. J Gastroenterol Hepatol 1995; 10: pp. 413-418.
6. Chang L., Chen Y.L., Kao M.C.: Intracranial metastasis of hepatocellular carcinoma: review of 45 cases. Surg Neurol 2004; 62: pp. 172-177.
7. Zornig C., Broelsch C.E.: Impact of staging on the treatment of hepatocellular carcinoma. Endoscopy 1993; 25: pp. 138-142.
8. Som P., Atkins H.L., Bandoypadhyay D., et. al.: A fluorinated glucose analog, 2-fluoro-2-deoxy-D-glucose [18F]: nontoxic tracer for rapid tumor detection. J Nucl Med 1980; 21: pp. 670-675.
9. Trojan J., Schroeder O., Raedle J., et. al.: Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am J Gastroenterol 1999; 94: pp. 3314-3319.
10. Puig S., Thiele D.J.: Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol 2002; 6: pp. 171-180.
11. Zhou B., Gitschier J.: hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci U S A 1997; 94: pp. 7481-7486.
12. Chelly J., Tumer Z., Tonnesen T., et. al.: Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat Genet 1993; 3: pp. 14-19.
13. Bull P.C., Thomas G.R., Rommens J.M., et. al.: The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet 1993; 5: pp. 327-337.
14. Klomp L.W., Lin S.J., Yuan D.S., et. al.: Identification and functional expression of HAH1, a novel human gene involved in copper homeostasis. J Biol Chem 1997; 272: pp. 9221-9226.
15. Amaravadi R., Glerum D.M., Tzagoloff A.: Isolation of a cDNA encoding the human homolog of COX17, a yeast gene essential for mitochondrial copper recruitment. Hum Genet 1997; 99: pp. 329-333.
16. Culotta V.C., Klomp L.W.J., Strain J.: The copper chaperone for superoxide dismutase. J Biol Chem 1997; 199: pp. 23469-23472.
17. Theophanides T., Anastassopoulou J.: Copper and carcinogenesis. Crit Rev Oncol Hematol 2002; 1: pp. 57-64.
18. Margalioth E.J., Schenker J.G., Chevion M.: Copper and zinc levels in normal and malignant tissues. Cancer 1983; 5: pp. 868-872.
19. Diez M., Arroyo M., Cerdan F.J., et. al.: Serum and tissue trace metal levels in lung cancer. Oncology 1989; 4: pp. 230-234.
20. Peng F., Liu J., Wu J.S., et. al.: Mouse extra-hepatic hepatoma detected on microPET using copper (II)-64 chloride uptake mediated by endogenous mouse copper transporter 1. Mol Imaging Biol 2005; 7: pp. 325-329.
21. Peng F., Lu X., Janisse J., et. al.: Positron emission tomography of human prostate cancer xenografts in mice with increased uptake of copper (II)-64 chloride. J Nucl Med 2006; 47: pp. 1649-1652.
22. Hudson H.M., Larkin R.S.: Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging 1994; 13: pp. 601-609.
23. Woodard H.Q., Gigler R.E., Freed B., et. al.: Expression of tissue isotope distribution. J Nucl Med 1975; 16: pp. 958-959.
24. Xie D., Gore C., Liu J., et. al.: Role of DAB2IP in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis. Proc Natl Acad Sci U S A 2010; 107: pp. 2485-2490.
25. Osborn S.B., Szaz K.F., Walshe J.M.: Studies with radioactive copper ( 64 Cu and 67 Cu): abdominal scintiscans in patients with Wilson’s disease. Q J Med 1969; 38: pp. 467-474.
26. Walshe J.M., Potter G.: The pattern of the whole body distribution of radioactive copper ( 67 Cu, 64 Cu) in Wilson’s disease and various control groups. Q J Med 1977; 46: pp. 445-462.
27. Skromne-Kadlubik G., Diaz J.F., Celis C.: Basal ganglia scans in the human. J. Nucl Med 1975; 16: pp. 787-788.
28. Sternlieb I., Scheinberg I.H.: Radiocopper in diagnosing liver disease. Semin Nucl Med 1972; 2: pp. 176-188.
29. Blower P.J., Lewis J.S., Zweit J.: Copper radionuclides and radiopharmaceuticals in nuclear medicine. Nucl Med Biol 1996; 23: pp. 957-980.
30. Peng F, Lutsenko S, Sun X, et al. Positron emission tomography of copper metabolism in the Atp7b−/− knock-out mouse model of Wilson’s disease. Mol Imaging Biol. In press.
31. Holzer A.K., Varki N.M., Le Q.T., et. al.: Expression of the human copper influx transporter 1 in normal and malignant human tissues. J Histochem Cytochem 2006; 54: pp. 1041-1049.
32. Yoshi J., Yoshi H., Kuriyama S., et. al.: The copper-chelating agent, trientine, suppresses tumor development and angiogenesis in the murine hepatocellular carcinoma cells. Int J Cancer 2001; 94: pp. 768-773.
33. Moriguchi M., Nakajima T., Kimura H., et. al.: The copper chelator trientine has an antiangiogenic effect against hepatocellular carcinoma, possibly through inhibition of interleukin-8 production. Int J Cancer 2002; 102: pp. 445-452.