Rationale and Objectives
To detect whether the efficacy of microwave ablation (MWA) could be improved by preinjected fluids in an ex vivo porcine liver model.
Materials and Methods
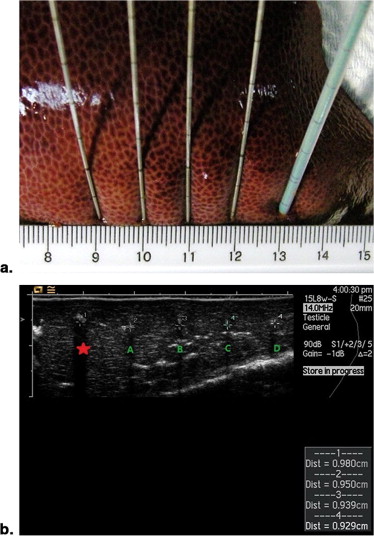
Ablations were performed for 12 minutes using energy output of impedance-based (power output gradually rose to 200W, maintained until increases in tissue impedance of 20 Ω, reduced to 10W, and switched on again 15 seconds later) in radiofrequency ablation (RFA) or 80 W in MWA. Before ablation, 5 mL of ethanol, distilled water, 0.9% NaCl solution, or 10% NaCl solution ( n = 6 each) was injected into the targeted liver tissue. Ablations without fluid injection served as control. The ablation diameter, volume, shape index, and temperature were recorded and compared.
Results
Preinjection of 0.9% or 10% NaCl solution resulted in larger coagulation volumes than that of the control group in RFA experiments (28.1 ± 2.9 cm 3 , 45.3 ± 6.3 cm 3 , 20.0 ± 2.5 cm 3 , respectively; P < .05). Ethanol and distilled water had no impact on coagulation volumes in RFA. Preinjection of ethanol or 10% NaCl solution created smaller coagulation volumes than that of the control group in MWA experiments (34.3 ± 2.0 cm 3 , 33.9 ± 4.1 cm 3 , 58.0 ± 6.6 cm 3 , respectively; P < .001). 0.9% NaCl solution and distilled water had no impact on coagulation volumes in MWA.
Conclusion
In an ex vivo porcine liver, preinjected fluids do not benefit microwave ablation as those in radiofrequency ablation.
Image-guided microwave ablation (MWA) and radiofrequency ablation (RFA) are two widely used thermal ablation therapies to eliminate primary or metastatic liver tumors, especially for patients who are not candidates for surgical resection or liver transplantation . In RFA, a high-frequency alternating electric current (375–500 kHz) is used to create ionic flow, which produces frictional heat and heat conduction to induce tissue necrosis. Tissue electrical and thermal conductivities are important during RFA . In contrast, MWA involves the application of electromagnetic field by perturbing polar molecules (primarily H 2 O) in tissue. Tissues with high water content readily absorb microwave .
Currently, thermal ablation therapy is being developed to yield a large area of controlled coagulation necrosis with a single application of energy. Several strategies have been developed to increase the amount of coagulation necrosis, including increasing energy deposition, modulating tissue characteristics, and modifying tissue blood flow . RFA efficacy was shown to be improved when tissue was injected with ethanol or NaCl solution prior to, or during the ablation procedure . In MWA, however, the mechanisms for increasing ablative coagulation may not necessarily be the same, because heating relies on a different mechanism. To improve MWA, the question of whether preinjected liquids improve ablation should be explored independently. The purpose of this study was to detect whether the efficacy of MWA could be improved by preinjected fluids, as those in RFA.
Material and methods
Ablation Systems
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Ablation Experiments
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Table 1
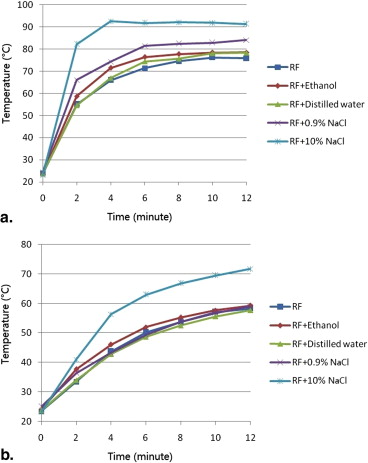
Mean Diameter, Volume, Shape Index, and Temperature of RFA Experiments
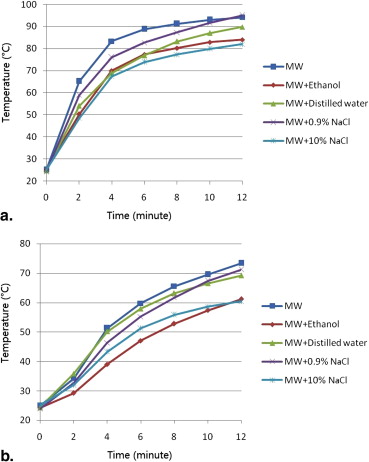
Parameters Control Preinjected Fluids Ethanol Distilled Water 0.9% NaCl Solution 10% NaCl Solution Coagulation diameter (cm) Long-axis 3.9 ± 0.2 4.1 ± 0.3 4.1 ± 0.2 4.1 ± 0.2 4.8 ± 0.1 ∗ Short-axis 3.1 ± 0.1 3.3 ± 0.2 3.3 ± 0.2 3.6 ± 0.1 ∗ 4.2 ± 0.3 ∗ † Dl/Ds ratio 1.26 ± 0.06 1.24 ± 0.07 1.26 ± 0.10 1.15 ± 0.05 ∗ 1.14 ± 0.05 ∗ Volume (cm 3 ) 20.0 ± 2.5 23.5 ± 3.3 22.9 ± 2.5 28.1 ± 2.9 ∗ 45.3 ± 6.3 ∗ † Maximum temperature (°C) Probe A 76.8 ± 8.2 81.1 ± 8.2 80.0 ± 8.5 86.7 ± 6.7 ∗ 97.3 ± 5.9 ∗ † Probe B 58.8 ± 7.4 59.5 ± 9.7 57.8 ± 7.4 59.2 ± 5.4 71.9 ± 8.5 ∗ Time to 54°C, probe A (seconds) 135.0 ± 33.9 110.0 ± 35.2 130.0 ± 39.0 88.3 ± 33.1 ∗ 61.7 ± 22.3 ∗
Six ablations were performed for each group. Data are the mean values ± standard deviation.
Dl, long-axis diameter; Ds, short-axis diameters; RFA, radiofrequency ablation.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 2
Mean Diameter, Volume, Shape Index, and Temperature of MWA Experiments
Parameters Control Preinjected Fluids Ethanol Distilled Water 0.9% NaCl solution 10% NaCl Solution Coagulation diameter (cm) Long-axis 5.9 ± 0.2 5.2 ± 0.2 ∗ 6.0 ± 0.2 5.8 ± 0.2 5.3 ± 0.2 ∗ Short-axis 4.3 ± 0.2 3.6 ± 0.1 ∗ 4.3 ± 0.2 4.2 ± 0.2 3.5 ± 0.1 ∗ Dl/Ds ratio 1.36 ± 0.08 1.47 ± 0.08 ∗ 1.40 ± 0.08 1.38 ± 0.04 1.53 ± 0.03 ∗ Volume (cm 3 ) 58.0 ± 6.6 34.3 ± 2.0 ∗ 56.5 ± 5.0 54.5 ± 6.8 33.9 ± 4.1 ∗ Maximum temperature (°C) Probe A 94.2 ± 6.3 84.3 ± 9.8 ∗ 89.8 ± 6.1 95.0 ± 5.8 82.0 ± 8.6 ∗ Probe B 73.4 ± 8.6 61.2 ± 7.3 ∗ 69.2 ± 8.4 71.2 ± 6.9 60.4 ± 8.3 ∗ Time to 54°C, probe A (seconds) 98.3 ± 35.4 145.0 ± 39.9 ∗ 133.3 ± 32.0 116.7 ± 37.2 163.3 ± 38.3 ∗
Six ablations were performed for each group. Data are the mean values ± standard deviation.
Dl, long-axis diameter; Ds, short-axis diameters; MWA, microwave ablation.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
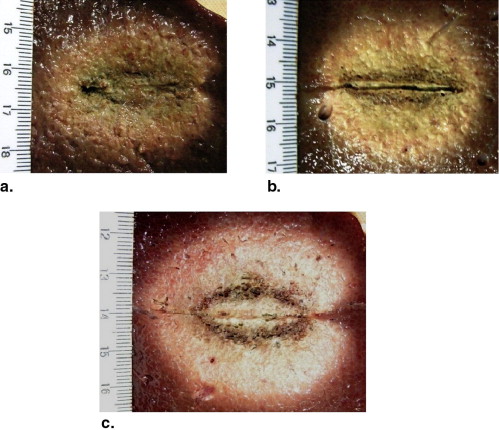
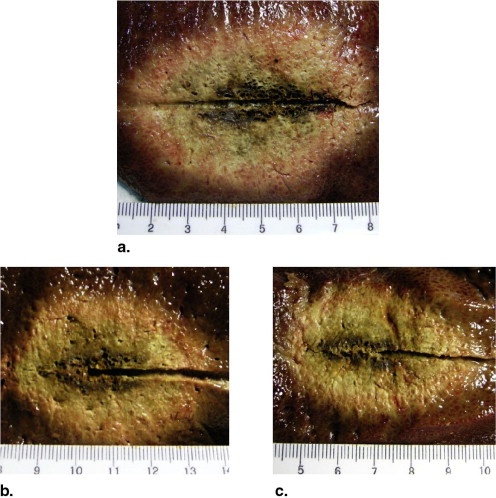
RFA
Get Radiology Tree app to read full this article<
Microwave Ablation
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Kuang M., Lu M.D., Xie X.Y., et. al.: Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna—experimental and clinical studies. Radiology 2007; 242: pp. 914-924.
2. Liang P., Wang Y., Yu X., et. al.: Malignant liver tumors: treatment with percutaneous microwave ablation—complications among cohort of 1136 patients. Radiology 2009; 251: pp. 933-940.
3. Huang J., Hernandez-Alejandro R., Croome K.P., et. al.: Radiofrequency ablation versus surgical resection for hepatocellular carcinoma in childs a cirrhotics-a retrospective study of 1,061 cases. J Gastrointest Surg 2010; 15: pp. 311-320.
4. Peng Z.W., Zhang Y.J., Chen M.S., et. al.: Radiofrequency ablation as first-line treatment for small solitary hepatocellular carcinoma: long-term results. Eur J Surg Oncol 2010; 36: pp. 1054-1060.
5. Brace C.L.: Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences?. Curr Probl Diagn Radiol 2009; 38: pp. 135-143.
6. Lubner M.G., Brace C.L., Hinshaw J.L., et. al.: Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 2010; 21: pp. S192-S203.
7. Ahmed M., Brace C.L., Lee F.J., et. al.: Principles of and advances in percutaneous ablation. Radiology 2011; 258: pp. 351-369.
8. Vallone P., Catalano O., Izzo F., et. al.: Combined ethanol injection therapy and radiofrequency ablation therapy in percutaneous treatment of hepatocellular carcinoma larger than 4 cm. Cardiovasc Intervent Radiol 2006; 29: pp. 544-551.
9. Aubé C., Schmidt D., Brieger J., et. al.: Influence of NaCl concentrations on coagulation, temperature, and electrical conductivity using a perfusion radiofrequency ablation system an ex vivo experimental study. Cardiovasc Intervent Radiol 2007; 30: pp. 92-97.
10. Shimizu A., Ishizaka H., Awata S., et. al.: Expansion of radiofrequency ablation volume by saturated NaCl saline injection in the area of vaporization. Acta Radiol 2009; 50: pp. 61-64.
11. Schirmang T.C., Dupuy D.E.: Image-guided thermal ablation of nonresectable hepatic tumors using the Cool-Tip radiofrequency ablation system. Expert Rev Med Devices 2007; 4: pp. 803-814.
12. Yu J., Liang P., Yu X., et. al.: A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: Results in ex vivo and in vivo porcine livers. Eur J Radiol 2011; 79: pp. 124-130.
13. Dong B.W., Liang P., Yu X.L., et. al.: Sonographically guided microwave coagulation treatment of liver cancer: an experimental and clinical study. AJR Am J Roentgenol 1998; 171: pp. 449-454.
14. Godlewski G., Rouy S., Pignodel C., et. al.: Deep localized neodymium (Nd)-YAG laser photocoagulation in liver using a new water cooled and echoguided handpiece. Lasers Surg Med 1988; 8: pp. 501-509.
15. Shi W, Liang P, Zhu Q, et al. Microwave ablation: results with double 915MHz antennae in ex vivo bovine Livers. Eur J Radiol 2010. In press.
16. Morimoto M., Sugimori K., Shirato K., et. al.: Treatment of hepatocellular carcinoma with radiofrequency ablation: radiologic-histologic correlation during follow-up periods. Hepatology 2002; 35: pp. 1467-1475.
17. Hänsler J., Frieser M., Tietz V., et. al.: Percutaneous radiofrequency ablation of liver tumors using multiple saline-perfused electrodes. J Vasc Interv Radiol 2007; 18: pp. 405-410.
18. Goldberg S.N., Ahmed M., Gazelle G.S., et. al.: Radio-frequency thermal ablation with NaCl solution injection: effect of electrical conductivity on tissue heating and coagulation-phantom and porcine liver study. Radiology 2001; 219: pp. 157-165.
19. Gillams A.R., Lees W.R.: CT mapping of the distribution of saline during radiofrequency ablation with perfusion electrodes. Cardiovasc Intervent Radiol 2005; 28: pp. 476-480.
20. Lobo S.M., Afzal K.S., Ahmed M., et. al.: Radiofrequency ablation: modeling the enhanced temperature response to adjuvant NaCl pretreatment. Radiology 2004; 230: pp. 175-182.
21. Bruners P., Muller H., Gunther R.W., et. al.: Fluid-modulated bipolar radiofrequency ablation: an ex-vivo evaluation study. Acta Radiol 2008; 49: pp. 258-266.
22. Kuang M., Lu M.D., Xie X.Y., et. al.: Ethanol ablation of hepatocellular carcinoma Up to 5.0 cm by using a multipronged injection needle with high-dose strategy. Radiology 2009; 253: pp. 552-561.
23. Kurokohchi K., Watanabe S., Masaki T., et. al.: Combination therapy of percutaneous ethanol injection and radiofrequency ablation against hepatocellular carcinomas difficult to treat. Int J Oncol 2002; 21: pp. 611-615.
24. Zhou P., Liu X., Li R., et. al.: Percutaneous coagulation therapy of hepatocellular carcinoma by combining microwave coagulation therapy and ethanol injection. Eur J Radiol 2009; 71: pp. 338-342.
25. Gao Y., Wang Y., Duan Y., et. al.: 915MHz microwave ablation with high output power in in vivo porcine spleens. Eur J Radiol 2010; 75: pp. 87-90.
26. Yoon J, Cho J, Kim N, et al. High frequency microwave ablation method for enhanced cancer treatment with minimized collateral damage. Int J Cancer 2010. In press.
27. Andreano A., Huang Y., Meloni M.F., et. al.: Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys 2010; 37: pp. 2967-2973.
28. Wright A.S., Sampson L.A., Warner T.F., et. al.: Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005; 236: pp. 132-139.