Rationale and Objectives
To test the ability of quantitative measures from preoperative dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) to predict, independently and/or with the Katz pathologic nomogram, which breast cancer patients with a positive sentinel lymph node biopsy will have four or more positive axillary lymph nodes on completion axillary dissection.
Materials and Methods
A retrospective review was conducted to identify clinically node-negative invasive breast cancer patients who underwent preoperative DCE-MRI, followed by sentinel node biopsy with positive findings and complete axillary dissection (June 2005–January 2010). Clinical/pathologic factors, primary lesion size, and quantitative DCE-MRI kinetics were collected from clinical records and prospective databases. DCE-MRI parameters with univariate significance ( P < .05) to predict four or more positive axillary nodes were modeled with stepwise regression and compared to the Katz nomogram alone and to a combined MRI–Katz nomogram model.
Results
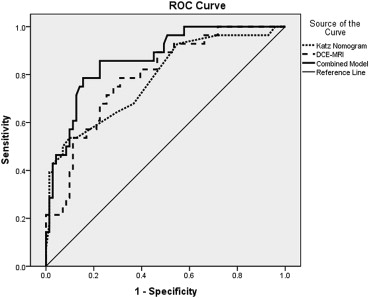
Ninety-eight patients with 99 positive sentinel biopsies met study criteria. Stepwise regression identified DCE-MRI total persistent enhancement and volume adjusted peak enhancement as significant predictors of four or more metastatic nodes. Receiver operating characteristic curves demonstrated an area under the curve of 0.78 for the Katz nomogram, 0.79 for the DCE-MRI multivariate model, and 0.87 for the combined MRI–Katz model. The combined model was significantly more predictive than the Katz nomogram alone ( P = .003).
Conclusions
Integration of DCE-MRI primary lesion kinetics significantly improved the Katz pathologic nomogram accuracy to predict the presence of metastases in four or more nodes. DCE-MRI may help identify sentinel node–positive patients requiring further local-regional therapy.
For breast cancer patients without clinical axillary lymph node involvement, sentinel lymph node biopsy has become the standard of care for assessing local-regional extent of the disease. It is well agreed on that patients with negative sentinel sampling need no further axillary surgery . However, which patients may avoid completion axillary dissection after positive sentinel sampling is an evolving consideration, especially in the setting of adjuvant radiotherapy. Furthermore, optimal design of radiotherapy fields with regard to regional lymphatic coverage is not known.
Very low rates of local-regional recurrence in select sentinel node–positive patients who forego axillary dissection in the setting of planned radiotherapy supports a movement away from completion axillary dissection in low-risk patients. However, low-risk criteria have not been precisely defined . The rationale behind the increasing tendency to avoid additional surgery is clear—completion axillary dissection carries a 10%–40% risk of lymphedema , and excellent local control of the axilla has been demonstrated in previous studies with radiation therapy alone .
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Pathology
Get Radiology Tree app to read full this article<
DCE-MRI Acquisition
Get Radiology Tree app to read full this article<
DCE-MRI Data Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
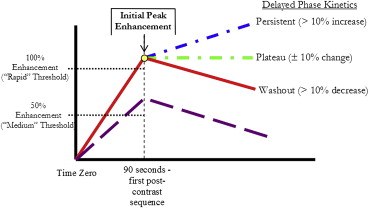
Early-phase kinetics
Get Radiology Tree app to read full this article<
Delayed-phase kinetics
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Table 1
Patient and Breast Cancer Pathologic Characteristics
Characteristics Mean ± Standard Deviation Range Patient age (years) 55.5 ± 10.4 31.5–75.4 Pathologic primary cancer size at the time of lumpectomy (cm) 3.2 ± 2.1 0.0–10.0 Number of positive sentinel lymph nodes 1.7 ± 1.3 1–11 Number of positive lymph nodes at completion dissection in patients with positive completion dissections 6.1 ± 7.3 1–29
Proportion Lobular histology 33/99 = 33% Estrogen receptor positive 94/99 = 95% Progesterone receptor positive 87/99 = 88% Her-2 positive 8/99 = 8% Nottingham grade 1 24/99 = 24% 2 50/99 = 51% 3 25/99 = 25%
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 2
Univariate Analysis—Correlations with Presence of Four or More Positive Axillary Lymph Nodes
Parameter Metric OR (95% CI)P Value Primary lesion volume Per log increase 2.20 (1.1–4.2) .009 Initial peak enhancement 2.12 (0.4–11.3) .32 Volume × peak 2.02 (1.1–3.7) .01 Percent rapid enhancement Per 10% increase 0.87 (0.7–1.0) .10 Percent rapid persistent enhancement 1.24 (0.9–1.7) .19 Percent rapid plateau enhancement 0.78 (0.5–1.1) .19 Percent rapid washout 0.66 (0.5–0.9) .005 Percent medium enhancement 1.21 (1.0–1.4) .02 Percent medium persistent enhancement 1.39 (1.2–1.7) .0003 Percent medium plateau enhancement 0.82 (0.5–1.3) .35 Percent medium washout 0.59 (0.3–1.2) .09 Total percent persistent enhancement 1.49 (1.2–1.8) <.0001 Total percent plateau enhancement 0.77 (0.6–1.1) .09 Total percent washout 0.69 (0.5–0.9) .002
CI, confidence interval; OR, odds ratio.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Krag D.N., Anderson S.J., Julian T.B., et. al.: Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 2010; 11: pp. 927-933.
2. Giuliano A.E., McCall L., Beitsch P., et. al.: Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 2010; 252: pp. 426-432.
3. Giuliano A.E., Hunt K.K., Ballman K.V., et. al.: Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011; 305: pp. 569-575.
4. Giuliano A.E., Chung A.P.: Long-term follow-up confirms the oncologic safety of sentinel node biopsy without axillary dissection in node-negative breast cancer patients. Ann Surg 2010; 251: pp. 601-603.
5. Van Zee K.J., Manasseh D.M., Bevilacqua J.L., et. al.: A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol 2003; 10: pp. 1140-1151.
6. Park J., Fey J.V., Naik A.M., et. al.: A declining rate of completion axillary dissection in sentinel lymph node-positive breast cancer patients is associated with the use of a multivariate nomogram. Ann Surg 2007; 245: pp. 462-468.
7. Veronesi U., Orecchia R., Zurrida S., et. al.: Avoiding axillary dissection in breast cancer surgery: a randomized trial to assess the role of axillary radiotherapy. Ann Oncol 2005; 16: pp. 383-388.
8. Kuehn T., Klauss W., Darsow M., et. al.: Long-term morbidity following axillary dissection in breast cancer patients—clinical assessment, significance for life quality and the impact of demographic, oncologic and therapeutic factors. Breast Cancer Res Treat 2000; 64: pp. 275-286.
9. Schijven M.P., Vingerhoets A.J., Rutten H.J., et. al.: Comparison of morbidity between axillary lymph node dissection and sentinel node biopsy. Eur J Surg Oncol 2003; 29: pp. 341-350.
10. Norman S.A., Localio A.R., Potashnik S.L., et. al.: Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol 2009; 27: pp. 390-397.
11. Fisher B., Jeong J.H., Anderson S., et. al.: Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 2002; 347: pp. 567-575.
12. Fowble B., Gray R., Gilchrist K., et. al.: Identification of a subgroup of patients with breast cancer and histologically positive axillary nodes receiving adjuvant chemotherapy who may benefit from postoperative radiotherapy. J Clin Oncol 1988; 6: pp. 1107-1117.
13. Katz A., Smith B.L., Golshan M., et. al.: Nomogram for the prediction of having four or more involved nodes for sentinel lymph node-positive breast cancer. J Clin Oncol 2008; 26: pp. 2093-2098.
14. Loiselle C.R., Eby P.R., DeMartini W.B., et. al.: Dynamic contrast-enhanced MRI kinetics of invasive breast cancer: a potential prognostic marker for radiation therapy. Int J Radiat Oncol Biol Phys 2010; 76: pp. 1314-1319.
15. Tuncbilek N., Karakas H.M., Okten O.O.: Dynamic magnetic resonance imaging in determining histopathological prognostic factors of invasive breast cancers. Eur J Radiol 2005; 53: pp. 199-205.
16. Mussurakis S., Buckley D.L., Horsman A.: Prediction of axillary lymph node status in invasive breast cancer with dynamic contrast-enhanced MR imaging. Radiology 1997; 203: pp. 317-321.
17. Partridge S.C., Gibbs J.E., Lu Y., et. al.: MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol 2005; 184: pp. 1774-1781.
18. Marinovich M.L., Sardanelli F., Ciatto S., et. al.: Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast 2012; 21: pp. 669-677.
19. Partridge S.C., DeMartini W.B., Kurland B.F., et. al.: Quantitative diffusion-weighted imaging as an adjunct to conventional breast MRI for improved positive predictive value. AJR Am J Roentgenol 2009; 193: pp. 1716-1722.
20. Wang L.C., DeMartini W.B., Partridge S.C., et. al.: MRI-detected suspicious breast lesions: predictive values of kinetic features measured by computer-aided evaluation. AJR Am J Roentgenol 2009; 193: pp. 826-831.
21. Eby P.R., Partridge S.C., White S.W., et. al.: Metabolic and vascular features of dynamic contrast-enhanced breast magnetic resonance imaging and (15)O-water positron emission tomography blood flow in breast cancer. Acad Radiol 2008; 15: pp. 1246-1254.
22. Eby P.R., DeMartini W.B., Gutierrez R.L., et. al.: Characteristics of probably benign breast MRI lesions. AJR Am J Roentgenol 2009; 193: pp. 861-867.
23. American College of Radiology Breast Magnetic Resonance Imaging (MRI) Accreditation Program. Available at: http://www.acr.org/Quality-Safety/Accreditation/BreastMRI . Accessed August 30, 2013.
24. Werkoff G., Lambaudie E., Fondrinier E., et. al.: Prospective multicenter comparison of models to predict four or more involved axillary lymph nodes in patients with breast cancer with one to three metastatic sentinel lymph nodes. J Clin Oncol 2009; 27: pp. 5707-5712.
25. Kvistad K.A., Rydland J., Smethurst H.B., et. al.: Axillary lymph node metastases in breast cancer: preoperative detection with dynamic contrast-enhanced MRI. Eur Radiol 2000; 10: pp. 1464-1471.
26. Scaranelo A.M., Eiada R., Jacks L.M., et. al.: Accuracy of unenhanced MR imaging in the detection of axillary lymph node metastasis: study of reproducibility and reliability. Radiology 2012; 262: pp. 425-434.
27. He N., Xie C., Wei W., et. al.: A new, preoperative, MRI-based scoring system for diagnosing malignant axillary lymph nodes in women evaluated for breast cancer. Eur J Radiol 2012; 81: pp. 2602-2612.
28. Zahra M.A., Hollingsworth K.G., Sala E., et. al.: Dynamic contrast-enhanced MRI as a predictor of tumour response to radiotherapy. Lancet Oncol 2007; 8: pp. 63-74.
29. Esserman L., Hylton N., George T., et. al.: Contrast-enhanced magnetic resonance imaging to assess tumor histopathology and angiogenesis in breast carcinoma. Breast J 1999; 5: pp. 13-21.
30. Buadu L.D., Murakami J., Murayama S., et. al.: Breast lesions: correlation of contrast medium enhancement patterns on MR images with histopathologic findings and tumor angiogenesis. Radiology 1996; 200: pp. 639-649.
31. Stomper P.C., Winston J.S., Herman S., et. al.: Angiogenesis and dynamic MR imaging gadolinium enhancement of malignant and benign breast lesions. Breast Cancer Res Treat 1997; 45: pp. 39-46.
32. Hulka C.A., Edmister W.B., Smith B.L., et. al.: Dynamic echo-planar imaging of the breast: experience in diagnosing breast carcinoma and correlation with tumor angiogenesis. Radiology 1997; 205: pp. 837-842.
33. Matsubayashi R., Matsuo Y., Edakuni G., et. al.: Breast masses with peripheral rim enhancement on dynamic contrast-enhanced MR images: correlation of MR findings with histologic features and expression of growth factors. Radiology 2000; 217: pp. 841-848.
34. Bartella L., Morris E.A., Dershaw D.D., et. al.: Proton MR spectroscopy with choline peak as malignancy marker improves positive predictive value for breast cancer diagnosis: preliminary study. Radiology 2006; 239: pp. 686-692.
35. Turnbull L., Brown S., Harvey I., et. al.: Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet 2010; 375: pp. 563-571.
36. Turnbull L.W., Brown S.R., Olivier C., et. al.: Multicentre randomised controlled trial examining the cost-effectiveness of contrast-enhanced high field magnetic resonance imaging in women with primary breast cancer scheduled for wide local excision (COMICE). Health Technol Assess 2010; 14: pp. 1-182.