Rationale and Objectives
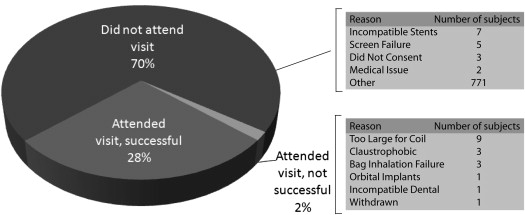
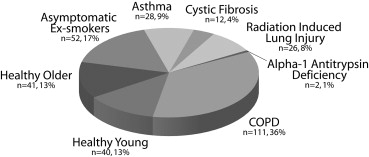
In this review, we summarize our experience evaluating pulmonary function in 330 different subjects using hyperpolarized noble gas magnetic resonance imaging (MRI) after enrollment and screening of >1100 subjects with and without respiratory disease during the period February 1, 2006, through November 1, 2012.
Materials and Methods
We discuss the feasibility of hyperpolarized gas MRI research in a small nonhospital research unit and provide an overview of our experience since we initiated patient-based studies. We also discuss the importance of infrastructure support, collaboration, research trainees, and a large and willing patient population that helped to advance the research and technological deliverables. A summary of patient safety and tolerability, key feasibility, and research milestones is provided, as well as a roadmap for future studies.
Results
Hyperpolarized 3 He and 129 Xe gas MRI is feasible at smaller centers without significant human resources for large and small longitudinal studies by virtue of its excellent patient safety and tolerability, the speed with which images can be acquired and quantitatively analyzed and the high spatial-temporal dynamics of the method that allows for acute and chronic therapy studies.
Conclusions
The hyperpolarized noble gas MRI community’s highly collaborative efforts and motivation to further the development and application of this tool has resulted in a moment-of-opportunity to translate the method clinically to provide an improved understanding of pulmonary disease. There are, as well, new and unprecedented opportunities for the evaluation of disease progression and to help develop the new treatments and interventions critically required for chronic pulmonary disease.
Magnetic resonance imaging (MRI) was developed as a research and diagnostic imaging tool to provide unique tissue contrast with high spatial and temporal resolution. In contrast to other diagnostic imaging methods, MRI does not require the use of x-rays or other ionizing radiation, offering the potential for intensive serial and longitudinal studies in the same patient, in children, and in radiation-sensitive tissues. However, despite these very practical advantages, MRI of the lung provides a unique challenge due to the low signal from the lung parenchyma because of the low proton ( 1 H) density (versus high gas density) in the lung, as well as the effects of motion, magnetic susceptibility, and the multiexponential T2 relaxation times .
With the understanding that image contrast in conventional 1 H MRI depends on the 1 H density of the tissue as well as the magnetic environment of the protons within that tissue, a number of MRI methods can be used for lung imaging, including ultra-short echo time (UTE) methods and Fourier decomposition . 1 H MRI contrast agents including oxygen-enhanced MRI have also been developed and demonstrated to provide novel regional, morphological, and functional measurements in respiratory disease .
Get Radiology Tree app to read full this article<
Figure 1
Hyperpolarized Helium-3 and Xenon-129 Magnetic Resonance Imaging 3 He MRI (green) and 129 Xe MRI (blue) static ventilation images registered to the 1 H MRI anatomical image for a representative healthy volunteer (age=75, sex=female, FEV 1 =93% pred ) and representative subjects with chronic obstructive pulmonary disease (age=77, sex=female, FEV 1 =50% pred ), cystic fibrosis (age=24, sex=female, FEV 1 =70% pred ), asthma (age=43, sex=male, FEV 1 =82% pred ) and radiation induced lung injury (age=66, sex=male, FEV 1 =70% pred ).
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
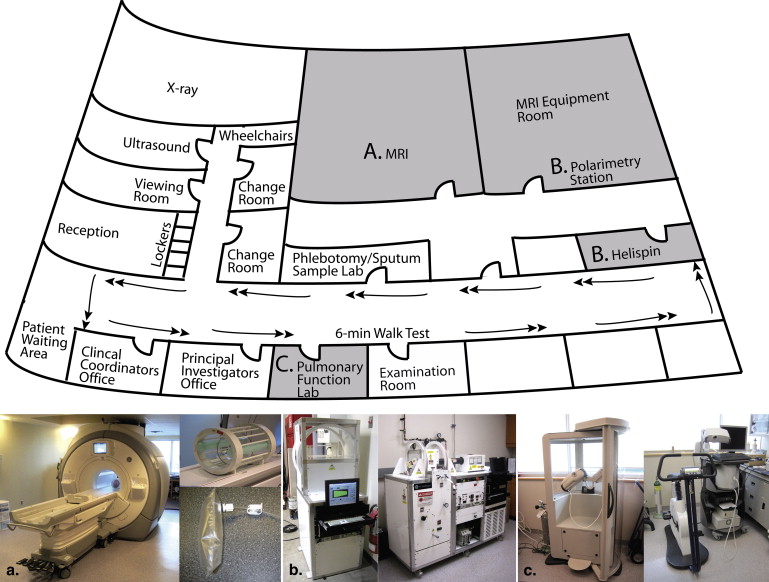
Unique facilities and infrastructure
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Collaborations: Local and International
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Motivated Research Subjects
Get Radiology Tree app to read full this article<
Table 1
Approved Clinical Trial Applications Summary
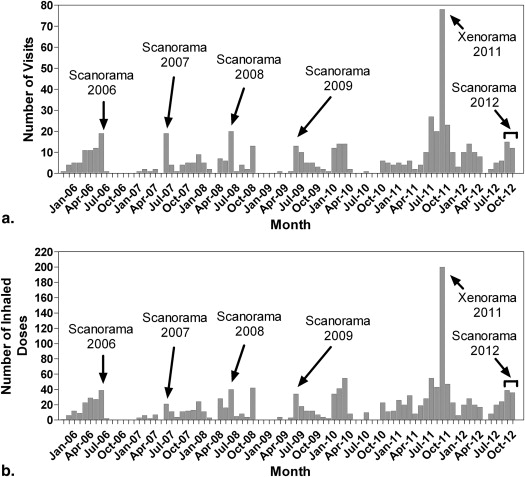
Protocol Number Protocol Name Year Start End Disease Longitudinal? 3 He Gas? 129 Xe Gas? ROB0003 Longitudinal 3 He Magnetic Resonance Imaging of Healthy Lung 2010 - HV Y Y N ROB0005 Hyperpolarized He Magnetic Resonance Imaging of Adult Patients with Cystic Fibrosis 2007 2010 CF N Y N ROB0006 Development of 3.0 Tesla MRI Hardware and Software for 3 He gas imaging of the Lung: Healthy Volunteer Development Study 2006 - HV Y Y N ROB0007 A Single-Center Pilot Study Exploring the Utility of Magnetic Resonance Imaging in Patients with COPD 2005 2006 HV/COPD N Y N ROB0008 Assessment of Radiation-Induced Pneumonitis in Breast and Lung Cancer Patients 2007 2008 RILI N Y N ROB0010 A Single-Center Pilot Study Exploring the Utility of Magnetic Resonance Imaging in Patients with Chronic Lung Disease 2007 - Non-Healthy Lung Y Y N ROB0014 A 3 Period, Double-Blind, Randomized Crossover Study to Evaluate the Effects of a Single Dose of Montelukast Compared with Placebo on Exercise-Induced Bronchoconstriction as Assessed by Hyperpolarized Gas Magnetic Resonance Imaging 2007 2008 Asthma N Y N ROB0016 Predictors of Radiation-Induced Lung Injury Using 3 He Magnetic Resonance Imaging 2009 2010 RILI N Y N ROB0017 Hyperpolarized Helium-3 Magnetic Resonance Ventilation Heterogeneity and Airway Hyperresponsiveness in Asthma 2009 2011 Asthma N Y N ROB0018 Longitudinal Study of Helium-3 Magnetic Resonance Imaging of COPD 2009 - COPD Y Y N ROB0030 Xenon-129 Magnetic Resonance Imaging of Healthy Subjects: Hardware and Software Development and Reproducibility 2011 - HV Y N Y ROB0031 A Single-Center Study Evaluating Hyperpolarized Xenon-129 Magnetic Resonance Imaging in Subjects with Chronic Lung Disease 2011 - Non-Healthy Lung Y N Y ROB0034 Imaging Pulmonary Exacerbations in Patients with Cystic Fibrosis 2012 - CF Y Y Y ROB0036 Longitudinal Study of Oscillatory Positive Expiratory Pressure (oPEP) in Stable COPD 2012 2012 COPD Y Y N
HV, healthy volunteers; RILI, radiation-induced lung injury; CF, cystic fibrosis.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 2
Total Number of Visits, Scans, and Hyperpolarized Gas Doses for all Subjects Who Completed Imaging
No. of Visits All Visits 1 Only >1 but ≤ 5 >5 but ≤ 10 >10 Subjects 312 229 67 13 3 Completed scans 726 294 268 112 52 Inhaled doses 1428 596 486 219 127
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 3
All Adverse Events
Type All ( N = 312) HV ( n = 81) AE ( n = 52) Asthma ( n = 28) COPD ( n = 111) AAT ( n = 2) CF ( n = 12) RILI ( n = 26) Total 109 9 11 22 44 0 12 11 Serious ∗ 0 0 0 0 0 0 0 0 Hypoxic † 95 8 11 20 34 0 11 11 Withdrawn ‡ 1 0 0 0 1 0 0 0 AE intensity for other adverse events § Mild 11 1 0 2 7 0 1 0 Moderate 2 0 0 0 2 0 0 0 Severe 0 0 0 0 0 0 0 0 Relationship ‖ 3 He/ 129 Xe related 47 6 0 12 17 0 5 7 Unrelated 61 3 11 10 26 0 7 4
HV, healthy volunteer; AE, asymptomatic ex-smoker; COPD, chronic obstructive pulmonary disease; ATT, α 1 -antitrypsin deficiency; CF, cystic fibrosis; RILI, radiation-induced lung injury.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Important Role of Trainees and Other Research Deliverables
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Important considerations for start-up
Get Radiology Tree app to read full this article<
Future Directions
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
Acknowledgments
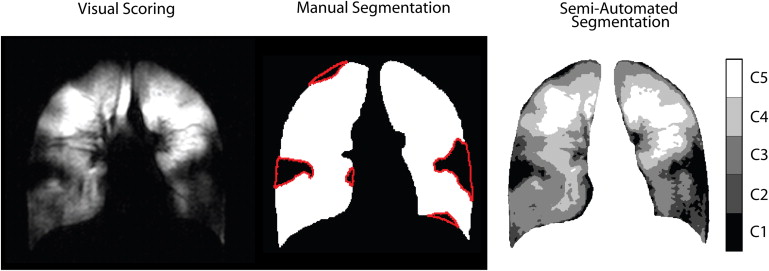
Get Radiology Tree app to read full this article<
References
1. Bergin C.J., Glover G.M., Pauly J.: Magnetic resonance imaging of lung parenchyma. J Thorac Imaging 1993; 8: pp. 12-17.
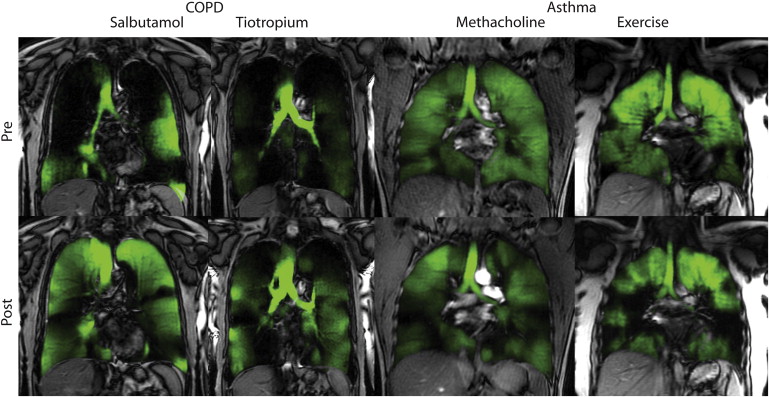
2. Muller N.L., Mayo J.R., Zwirewich C.V.: Value of MR imaging in the evaluation of chronic infiltrative lung diseases: comparison with CT. AJR Am J Roentgenol 1992; 158: pp. 1205-1209.
3. Mayo J.R., MacKay A., Muller N.L.: MR imaging of the lungs: value of short TE spin-echo pulse sequences. AJR Am J Roentgenol 1992; 159: pp. 951-956.
4. Deimling M, Jellus V, Geiger B et al. Time resolved lung ventilation imaging by Fourier decomposition. Proceedings of the 16th Annual Meeting of ISMRM, Toronto, Canada, 2639. 08. Abstract
5. Bauman G., Scholz A., Rivoire J., et. al.: Lung ventilation- and perfusion-weighted Fourier decomposition magnetic resonance imaging: in vivo validation with hyperpolarized (3) He and dynamic contrast-enhanced MRI. Magn Reson Med 2013; 69: pp. 229-237.
6. Bauman G., Lutzen U., Ullrich M., et. al.: Pulmonary functional imaging: qualitative comparison of Fourier decomposition MR imaging with SPECT/CT in porcine lung. Radiology 2011; 260: pp. 551-559.
7. Bauman G., Puderbach M., Deimling M., et. al.: Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of fourier decomposition in proton MRI. Magn Reson Med 2009; 62: pp. 656-664.
8. Edelman R.R., Hatabu H., Tadamura E., et. al.: Noninvasive assessment of regional ventilation in the human lung using oxygen-enhanced magnetic resonance imaging. Nat Med 1996; 2: pp. 1236-1239.
9. Chen Q., Jakob P.M., Griswold M.A., et. al.: Oxygen enhanced MR ventilation imaging of the lung. MAGMA 1998; 7: pp. 153-161.
10. Stock K.W., Chen Q., Morrin M., et. al.: Oxygen-enhanced magnetic resonance ventilation imaging of the human lung at 0.2 and 1.5 T. J Magn Reson Imaging 1999; 9: pp. 838-841.
11. Loffler R., Muller C.J., Peller M., et. al.: Optimization and evaluation of the signal intensity change in multisection oxygen-enhanced MR lung imaging. Magn Reson Med 2000; 43: pp. 860-866.
12. Ohno Y., Hatabu H., Takenaka D., et. al.: Oxygen-enhanced MR ventilation imaging of the lung: preliminary clinical experience in 25 subjects. AJR Am J Roentgenol 2001; 177: pp. 185-194.
13. Muller C.J., Schwaiblmair M., Scheidler J., et. al.: Pulmonary diffusing capacity: assessment with oxygen-enhanced lung MR imaging preliminary findings. Radiology 2002; 222: pp. 499-506.
14. Ohno Y., Hatabu H., Takenaka D., et. al.: Dynamic oxygen-enhanced MRI reflects diffusing capacity of the lung. Magn Reson Med 2002; 47: pp. 1139-1144.
15. Ohno Y., Koyama H., Yoshikawa T., et. al.: Comparison of capability of dynamic O(2)-enhanced MRI and quantitative thin-section MDCT to assess COPD in smokers. Eur J Radiol 2012; 81: pp. 1068-1075.
16. Ohno Y., Koyama H., Matsumoto K., et. al.: Oxygen-enhanced MRI vs. quantitatively assessed thin-section CT: pulmonary functional loss assessment and clinical stage classification of asthmatics. Eur J Radiol 2011; 77: pp. 85-91.
17. Ohno Y., Iwasawa T., Seo J.B., et. al.: Oxygen-enhanced magnetic resonance imaging versus computed tomography: multicenter study for clinical stage classification of smoking-related chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177: pp. 1095-1102.
18. Ohno Y., Koyama H., Nogami M., et. al.: Dynamic oxygen-enhanced MRI versus quantitative CT: pulmonary functional loss assessment and clinical stage classification of smoking-related COPD. AJR Am J Roentgenol 2008; 190: pp. W93-W99.
19. Jakob P.M., Wang T., Schultz G., et. al.: Assessment of human pulmonary function using oxygen-enhanced T(1) imaging in patients with cystic fibrosis. Magn Reson Med 2004; 51: pp. 1009-1016.
20. Kauczor H.U., Ebert M., Kreitner K.F., et. al.: Imaging of the lungs using 3He MRI: preliminary clinical experience in 18 patients with and without lung disease. J Magn Reson Imaging 1997; 7: pp. 538-543.
21. de Lange E.E., Mugler J.P., Brookeman J.R., et. al.: Lung air spaces: MR imaging evaluation with hyperpolarized 3He gas. Radiology 1999; 210: pp. 851-857.
22. Moller H.E., Chen X.J., Saam B., et. al.: MRI of the lungs using hyperpolarized noble gases. Magn Reson Med 2002; 47: pp. 1029-1051.
23. Albert M.S., Cates G.D., Driehuys B., et. al.: Biological magnetic resonance imaging using laser-polarized 129Xe. Nature 1994; 370: pp. 199-201.
24. Mugler J.P., Driehuys B., Brookeman J.R., et. al.: MR imaging and spectroscopy using hyperpolarized 129Xe gas: preliminary human results. Magn Reson Med 1997; 37: pp. 809-815.
25. Mugler JP, III, Mata JF, Wang HTJ, et al. The apparent diffusion coefficient of 129-Xe in the lung: preliminary human results. Proceedings of the 12th Annual Meeting of ISMRM, Kyoto, Japan, 769. 04. Abstract
26. Sindile A, Muradian I, Hrovat M et al. Human pulmonary diffusion weighted imaging at 0.2T with hyperpolarizd 129Xe. Proceedings of the 15th Annual Meeting of ISMRM, Berlin, Germany, 1290. 07. Abstract
27. Patz S., Hersman F.W., Muradian I., et. al.: Hyperpolarized (129)Xe MRI: a viable functional lung imaging modality?. Eur J Radiol 2007; 64: pp. 335-344.
28. Patz S., Muradian I., Hrovat M.I., et. al.: Human pulmonary imaging and spectroscopy with hyperpolarized 129Xe at 0.2T. Acad Radiol 2008; 15: pp. 713-727.
29. Cleveland Z.I., Cofer G.P., Metz G., et. al.: Hyperpolarized Xe MR imaging of alveolar gas uptake in humans. PLoS One 2010; 5: pp. e12192.
30. Mugler J.P., Altes T.A., Ruset I.C., et. al.: Simultaneous magnetic resonance imaging of ventilation distribution and gas uptake in the human lung using hyperpolarized xenon-129. Proc Natl Acad Sci U S A 2010; 107: pp. 21707-21712.
31. Kaushik S.S., Cleveland Z.I., Cofer G.P., et. al.: Diffusion-weighted hyperpolarized 129Xe MRI in healthy volunteers and subjects with chronic obstructive pulmonary disease. Magn Reson Med 2011; 65: pp. 1154-1165.
32. Dregely I., Mugler J.P., Ruset I.C., et. al.: Hyperpolarized Xenon-129 gas-exchange imaging of lung microstructure: first case studies in subjects with obstructive lung disease. J Magn Reson Imaging 2011; 33: pp. 1052-1062.
33. Driehuys B., Martinez-Jimenez S., Cleveland Z.I., et. al.: Chronic obstructive pulmonary disease: safety and tolerability of hyperpolarized 129Xe MR imaging in healthy volunteers and patients. Radiology 2012; 262: pp. 279-289.
34. Dregely I., Ruset I.C., Mata J.F., et. al.: Multiple-exchange-time xenon polarization transfer contrast (MXTC) MRI: initial results in animals and healthy volunteers. Magn Reson Med 2012; 67: pp. 943-953.
35. Driehuys B., Cofer G.P., Pollaro J., et. al.: Imaging alveolar-capillary gas transfer using hyperpolarized 129Xe MRI. Proc Natl Acad Sci U S A 2006; 103: pp. 18278-18283.
36. Lutey B.A., Lefrak S.S., Woods J.C., et. al.: Hyperpolarized 3He MR imaging: physiologic monitoring observations and safety considerations in 100 consecutive subjects. Radiology 2008; 248: pp. 655-661.
37. Ruset I.C., Ketel S., Hersman F.W.: Optical pumping system design for large production of hyperpolarized. Phys Rev Lett 2006; 96: pp. 053002.
38. Hersman F.W., Ruset I.C., Ketel S., et. al.: Large production system for hyperpolarized 129Xe for human lung imaging studies. Acad Radiol 2008; 15: pp. 683-692.
39. Driehuys B., Pollaro J., Cofer G.P.: In vivo MRI using real-time production of hyperpolarized 129Xe. Magn Reson Med 2008; 60: pp. 14-20.
40. Dregely I., Mugler J.P., Ruset I.C., et. al.: Hyperpolarized Xenon-129 gas-exchange imaging of lung microstructure: first case studies in subjects with obstructive lung disease. J Magn Reson Imaging 2011; 33: pp. 1052-1062.
41. Honda N., Osada H., Watanabe W., et. al.: Imaging of ventilation with dual-energy CT during breath hold after single vital-capacity inspiration of stable xenon. Radiology 2012; 262: pp. 262-268.
42. Chae E.J., Seo J.B., Goo H.W., et. al.: Xenon ventilation CT with a dual-energy technique of dual-source CT: initial experience. Radiology 2008; 248: pp. 615-624.
43. Carlson A.P., Brown A.M., Zager E., et. al.: Xenon-enhanced cerebral blood flow at 28% xenon provides uniquely safe access to quantitative, clinically useful cerebral blood flow information: a multicenter study. AJNR Am J Neuroradiol 2011; 32: pp. 1315-1320.
44. Suga K.: Technical and analytical advances in pulmonary ventilation SPECT with xenon-133 gas and Tc-99m-Technegas. Ann Nucl Med 2002; 16: pp. 303-310.
45. Shukla Y., Wheatley A., Kirby M., et. al.: Hyperpolarized (129)xe magnetic resonance imaging: tolerability in healthy volunteers and subjects with pulmonary disease. Acad Radiol 2012; 19: pp. 941-951.
46. Reis S, Ruppert K, Altes TA et al. Hyperpolarized Xe-129 CSI of the human lung: preliminary results from healthy, second-hand smoker and cystic fibrosis subjects. Proceedings of the 20th Annual Meeting of ISMRM, Melbourne, Australia, 629. 12. Ref Type: Abstract
47. Shukla Y., Wheatley A., Kirby M., et. al.: Hyperpolarized 129Xe magnetic resonance imaging: tolerability in healthy volunteers and subjects with pulmonary disease. Acad Radiol 2012; 19: pp. 941-951.
48. Altes T.A., Powers P.L., Knight-Scott J., et. al.: Hyperpolarized 3He MR lung ventilation imaging in asthmatics: preliminary findings. J Magn Reson Imaging 2001; 13: pp. 378-384.
49. Mathew L., Kirby M., Etemad-Rezai R., et. al.: Hyperpolarized (3)He magnetic resonance imaging: preliminary evaluation of phenotyping potential in chronic obstructive pulmonary disease. Eur J Radiol 2011; 79: pp. 140-146.
50. Kirby M., Mathew L., Wheatley A., et. al.: Chronic obstructive pulmonary disease: longitudinal hyperpolarized (3)He MR imaging. Radiology 2010; 256: pp. 280-289.
51. Mathew L., Gaede S., Wheatley A., et. al.: Detection of longitudinal lung structural and functional changes after diagnosis of radiation-induced lung injury using hyperpolarized 3He magnetic resonance imaging. Med Phys 2010; 37: pp. 22-31.
52. Mathew L., Evans A., Ouriadov A., et. al.: Hyperpolarized 3He magnetic resonance imaging of chronic obstructive pulmonary disease: reproducibility at 3.0 tesla. Acad Radiol 2008; 15: pp. 1298-1311.
53. Parraga G., Mathew L., Etemad-Rezai R., et. al.: Hyperpolarized 3He magnetic resonance imaging of ventilation defects in healthy elderly volunteers: initial findings at 3.0 Tesla. Acad Radiol 2008; 15: pp. 776-785.
54. Parraga G., Ouriadov A., Evans A., et. al.: Hyperpolarized 3He ventilation defects and apparent diffusion coefficients in chronic obstructive pulmonary disease: preliminary results at 3.0 Tesla. Invest Radiol 2007; 42: pp. 384-391.
55. Woodhouse N., Wild J.M., Paley M.N., et. al.: Combined helium-3/proton magnetic resonance imaging measurement of ventilated lung volumes in smokers compared to never-smokers. J Magn Reson Imaging 2005; 21: pp. 365-369.
56. de Lange E.E., Altes T.A., Patrie J.T., et. al.: The variability of regional airflow obstruction within the lungs of patients with asthma: assessment with hyperpolarized helium-3 magnetic resonance imaging. J Allergy Clin Immunol 2007; 119: pp. 1072-1078.
57. de Lange E.E., Altes T.A., Patrie J.T., et. al.: Evaluation of asthma with hyperpolarized helium-3 MRI: correlation with clinical severity and spirometry. Chest 2006; 130: pp. 1055-1062.
58. de Lange E.E., Altes T.A., Patrie J.T., et. al.: Changes in regional airflow obstruction over time in the lungs of patients with asthma: evaluation with 3He MR imaging. Radiology 2009; 250: pp. 567-575.
59. Mentore K., Froh D.K., de Lange E.E., et. al.: Hyperpolarized HHe 3 MRI of the lung in cystic fibrosis: assessment at baseline and after bronchodilator and airway clearance treatment. Acad Radiol 2005; 12: pp. 1423-1429.
60. Altes T.A., Rehm P.K., Harrell F., et. al.: Ventilation imaging of the lung: comparison of hyperpolarized helium-3 MR imaging with Xe-133 scintigraphy. Acad Radiol 2004; 11: pp. 729-734.
61. Fain S.B., Gonzalez-Fernandez G., Peterson E.T., et. al.: Evaluation of structure-function relationships in asthma using multidetector CT and hyperpolarized He-3 MRI. Acad Radiol 2008; 15: pp. 753-762.
62. Lee E.Y., Sun Y., Zurakowski D., et. al.: Hyperpolarized 3He MR imaging of the lung: normal range of ventilation defects and PFT correlation in young adults. J Thorac Imaging 2009; 24: pp. 110-114.
63. Kirby M., Heydarian M., Svenningsen S., et. al.: Hyperpolarized (3)he magnetic resonance functional imaging semiautomated segmentation. Acad Radiol 2012; 19: pp. 141-152.
64. Tustison N.J., Avants B.B., Flors L., et. al.: Ventilation-based segmentation of the lungs using hyperpolarized (3)He MRI. J Magn Reson Imaging 2011; 34: pp. 831-841.
65. Tustison N.J., Altes T.A., Song G., et. al.: Feature analysis of hyperpolarized helium-3 pulmonary MRI: a study of asthmatics versus nonasthmatics. Magn Reson Med 2010; 63: pp. 1448-1455.
66. Kirby M., Mathew L., Heydarian M., et. al.: Chronic obstructive pulmonary disease: quantification of bronchodilator effects by using hyperpolarized (3)He MR imaging. Radiology 2011; 261: pp. 283-292.
67. Costella S., Kirby M., Maksym G.N., et. al.: Regional pulmonary response to a methacholine challenge using hyperpolarized (3) He magnetic resonance imaging. Respirology 2012; 17: pp. 1237-1246.
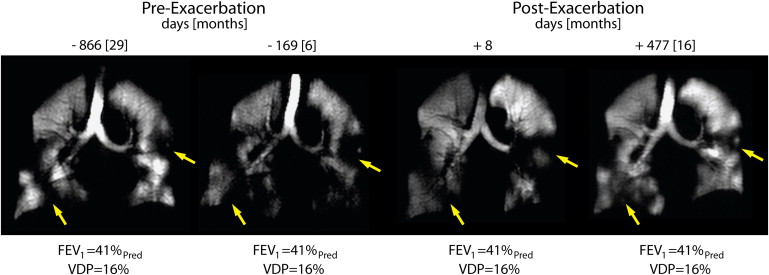
68. Kirby M., Kanhere N., Etemad-Rezai R., et. al.: Hyperpolarized helium-3 magnetic resonance imaging of chronic obstructive pulmonary disease exacerbation. J Magn Reson Imaging 2012; 37: pp. 1223-1227.
69. de Lange E.E., Altes T.A., Patrie J.T., et. al.: The variability of regional airflow obstruction within the lungs of patients with asthma: assessment with hyperpolarized helium-3 magnetic resonance imaging. J Allergy Clin Immunol 2007; 119: pp. 1072-1078.