Rationale and Objectives
The blood perfusion of peripheral nerves plays an important role in regeneration after nerve injury. Previous studies have shown that it is possible to quantitatively assess the blood perfusion of the tissue using contrast-enhanced ultrasound (CEUS). The aim of this study was to evaluate the feasibility of CEUS for quantitative assessment of the blood perfusion of the sciatic nerve in normal New Zealand white rabbits and to compare these parameters to those of surrounding skeletal muscle and the main artery in the thigh.
Materials and Methods
CEUS of the bilateral sciatic nerves was performed in 12 normal New Zealand white rabbits after a bolus injection of SonoVue (0.13 mL/kg). Pulse-inversion harmonic imaging was used for real-time CEUS. The blood perfusion of the left sciatic nerve was compared to that of its surrounding muscle, the arterial branch in the thigh, and the contralateral side.
Results
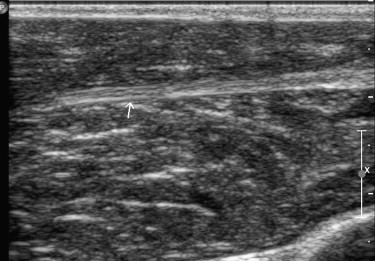
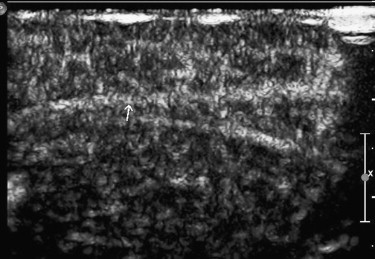
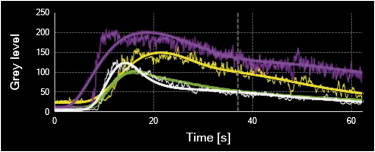
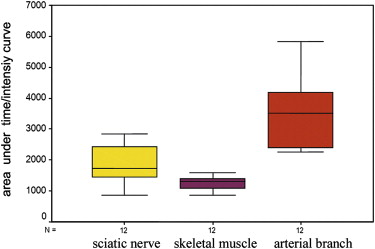
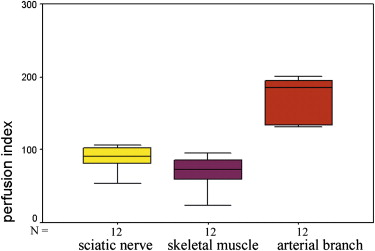
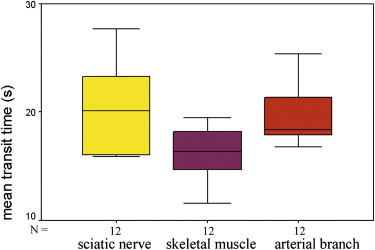
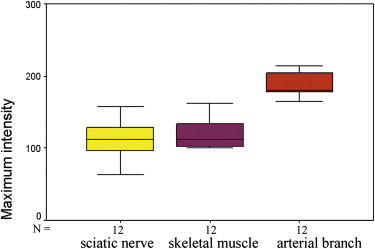
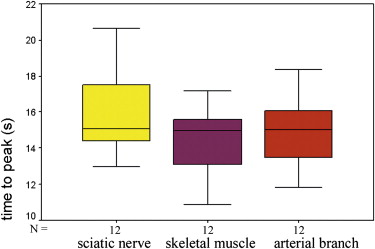
The supplying arteries in the sciatic nerve could be demonstrated during the early phase of CEUS, followed by the homogeneous enhancement of the whole nerve. The area under the curve and the perfusion index of the sciatic nerve were higher than those of the surrounding muscle and lower than those of the arterial branch in the thigh (both P values = .000). The maximum intensity of the sciatic nerve was similar to that of skeletal muscle and lower than that of the arterial branch. The time to peak was not significantly different among the sciatic nerve, skeletal muscle, and arterial branch ( P = .551). There were no differences in area under the curve, mean transit time, perfusion index, maximum intensity, and time to peak between the left and right sciatic nerves ( P > .05).
Conclusions
CEUS may be a feasible method for the quantitative assessment of blood perfusion of the peripheral nerves.
Blood perfusion of the peripheral nerves plays an important role in regeneration after nerve injury. First, vascularized nerve grafts have been shown to be superior to nonvascularized nerve grafts with respect to healing, especially when used in the hypovascular and scarred recipient bed . Because of the presence of sufficient and uninterrupted blood supply to the nerve graft, Schwann cell viability could be high, which would result in successful nerve healing. Second, cytokines and gene therapy have been tried to promote the angiogenesis of peripheral nerves, and the results were promising . Using nerve grafts pretreated with vascular endothelial growth factor, both invasion of Schwann cells and neovascularization could be promoted, which are important during nerve regeneration . Studies using chambers containing vascular endothelial growth factor in a laminin-based gel to repair rat sciatic nerve defects also demonstrated an overall relationship between increased vascularization and enhanced nerve regeneration .
The current techniques that have been used to evaluate peripheral nerve blood perfusion include radioactive microspheres, microelectrode hydrogen clearance polarography, and laser Doppler flowmetry . However, these methods are not suitable for the noninvasive assessment of blood perfusion in the peripheral nerves in clinical practice. Because of its lower sensitivity for the detection of slow blood flow, color and power Doppler ultrasound cannot display the microcirculation in the tissue. Contrast-enhanced ultrasound (CEUS), which uses gas-filled microbubbles as the tracer in the bloodstream, can dynamically display the entire microcirculation, including the tiny vascular branches supplying organs and tissues and the related capillary network below the spatial resolution of transducer (in the form of tissue enhancement) . When combined with targeted microbubbles, CEUS can also provide a promising noninvasive tool to quantify the early angiogenesis of tumors and may provide insights into the biology of tumor angiogenesis . Yet few studies have been performed using CEUS to evaluate the blood perfusion of the peripheral nerves. We hypothesized that CEUS could be used to enhance the peripheral nerves and to provide quantitative information about the nerves’ blood perfusion. Thus, this study was performed to evaluate the feasibility of CEUS for quantitative assessment of the blood perfusion of the sciatic nerve in normal New Zealand white rabbits.
Materials and methods
Animals
Get Radiology Tree app to read full this article<
Sonographic Contrast Agent
Get Radiology Tree app to read full this article<
Equipment and CEUS Examination
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Offline Analysis
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Vargel I., Demirci M., Erdem S., et. al.: A comparison of various vascularization-perfusion venous nerve grafts with conventional nerve grafts in rats. J Reconstr Microsurg 2009; 25: pp. 425-437.
2. Vargel I.: Impact of vascularization type on peripheral nerve microstructure. J Reconstr Microsurg 2009; 25: pp. 243-253.
3. Rovak J.M., Mungara A.K., Aydin M.A., et. al.: Effects of vascular endothelial growth factor on nerve regeneration in acellular nerve grafts. J Reconstr Microsurg 2004; 20: pp. 53-58.
4. Wongtrakul S., Bishop A.T., Friedrich P.F.: Vascular endothelial growth factor promotion of neoangiogenesis in conventional nerve grafts. J Hand Surg Am 2002; 27: pp. 277-285.
5. Pan H.C., Wu H.T., Cheng F.C., et. al.: Potentiation of angiogenesis and regeneration by G-CSF after sciatic nerve crush injury. Biochem Biophys Res Commun 2009; 382: pp. 177-182.
6. Hobson M.I., Green C.J., Terenghi G.: VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat 2000; 197: pp. 591-605.
7. Sondell M., Lundborg G., Kanje M.: Vascular endothelial growth factor stimulates Schwann cell invasion and neovascularization of acellular nerve grafts. Brain Res 1999; 846: pp. 219-228.
8. Hobson M.I.: Increased vascularisation enhances axonal regeneration within an acellular nerve conduit. Ann R Coll Surg Engl 2002; 84: pp. 47-53.
9. Maki Y., Firrell J.C., Breidenbach W.C.: Blood flow in mobilized nerves: results in a rabbit sciatic nerve model. Plast Reconstr Surg 1997; 100: pp. 627-633.
10. Höke A., Sun H.S., Gordon T., et. al.: Do denervated peripheral nerve trunks become ischemic? The impact of chronic denervation on vasa nervorum. Exp Neurol 2001; 172: pp. 398-406.
11. Jou I.M., Lai K.A., Shen C.L., et. al.: Changes in conduction, blood flow, histology, and neurological status following acute nerve-stretch injury induced by femoral lengthening. J Orthop Res 2000; 18: pp. 149-155.
12. Zhang W.Z., Zha D.G., Cheng G.X., et. al.: Assessment of regional myocardial blood flow with myocardial contrast echocardiography: an experimental study. Echocardiography 2004; 21: pp. 409-416.
13. Kalantarinia K., Belcik J.T., Patrie J.T., et. al.: Real-time measurement of renal blood flow in healthy subjects using contrast-enhanced ultrasound. Am J Physiol Renal Physiol 2009; 297: pp. F1129-F1134.
14. Ellegala D.B., Leong-Poi H., Carpenter J.E., et. al.: Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v)beta3. Circulation 2003; 108: pp. 336-341.
15. Leong-Poi H., Christiansen J., Klibanov A.L., et. al.: Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to alpha(v)-integrins. Circulation 2003; 107: pp. 455-460.
16. Stieger S.M., Dayton P.A., Borden M.A., et. al.: Imaging of angiogenesis using cadence contrast pulse sequencing and targeted contrast agents. Contrast Media Mol Imaging 2008; 3: pp. 9-18.
17. Giorgio A., De Stefano G., Coppola C., et. al.: Contrast-enhanced sonography in the characterization of small hepatocellular carcinomas in cirrhotic patients: comparison with contrast-enhanced ultrafast magnetic resonance imaging. Anticancer Res 2007; 27: pp. 4263-4269.
18. Lu M.D., Yu X.L., Li A.H., et. al.: Comparison of contrast enhanced ultrasound and contrast enhanced CT or MRI in monitoring subcutaneous thermal ablation procedure in patients with hepatocellular carcinoma: a multi-center study in China. Ultrasound Med Biol 2007; 33: pp. 1736-1749.
19. Cosgrove D., Eckersley R., Blomley M., et. al.: Quantification of blood flow. Eur Radiol 2001; 11: pp. 1338-1344.
20. Thijssen J.M., de Korte C.L.: Modeling ultrasound contrast measurement of blood flow and perfusion in biological tissue. Ultrasound Med Biol 2005; 31: pp. 279-285.
21. Rissanen T.T., Korpisalo P., Karvinen H., et. al.: High-resolution ultrasound perfusion imaging of therapeutic angiogenesis. JACC Cardiovasc Imaging 2008; 1: pp. 83-91.
22. Du J., Li F.H., Fang H., et. al.: Correlation of real-time gray scale contrast-enhanced ultrasonography with microvessel density and vascular endothelial growth factor expression for assessment of angiogenesis in breast lesions. J Ultrasound Med 2008; 27: pp. 821-831.
23. Weber M.A., Krix M., Jappe U., et. al.: Pathologic skeletal muscle perfusion in patients with myositis: detection with quantitative contrast-enhanced US—initial results. Radiology 2006; 238: pp. 640-649.
24. Lin C.C., Ding H.J., Chen Y.W., et. al.: Usefulness of thallium-201 muscle perfusion scan to investigate perfusion reserve in the lower limbs of type 2 diabetic patients. J Diabetes Complications 2004; 18: pp. 233-236.
25. Galbraith S.M., Lodge M.A., Taylor N.J., et. al.: Reproducibility of dynamic contrast-enhanced MRI in human muscle and tumours: comparison of quantitative and semi-quantitative analysis. NMR Biomed 2002; 15: pp. 132-142.
26. Krix M., Kiessling F., Vosseler S., et. al.: Comparison of intermittent-bolus contrast imaging with conventional power Doppler sonography: quantification of tumour perfusion in small animals. Ultrasound Med Biol 2003; 29: pp. 1093-1103.
27. Wang Z., Tang J., An L., et. al.: Contrast-enhanced ultrasonography for assessment of tumor vascularity in hepatocellular carcinoma. J Ultrasound Med 2007; 26: pp. 757-762.
28. Klauser A., Demharter J., De Marchi A., et. al.: Contrast-enhanced gray-scale sonography in assessment of joint vascularity in rheumatoid arthritis: results from the IACUS study group. Eur Radiol 2005; 15: pp. 2404-2410.