Rationale and Objectives
The purpose of this study was to evaluate prospectively a gadolinium-based perfusion technique for intrarenal blood flow in transplanted kidneys and to determine if magnetic resonance imaging (MRI) measurements of intrarenal perfusion could be used to differentiate between normal-functioning kidney allografts and allografts with acute tubular necrosis (ATN) or acute rejection.
Materials and Methods
Twenty-one subjects were enrolled within 4 months of receiving a kidney transplant. A biopsy was performed on subjects to diagnose each allograft as having either ATN or acute rejection. A group of subjects with normal functioning transplants was also enrolled in our study. MRI perfusion images were acquired on a 1.5 T MRI system within 48 hours after biopsy using an echo planar, T2∗-weighted sequence, and an injection of gadodiamide contrast agent administered at a dose of 0.1 mmol/kg. Scan parameters were: repetition time/echo time/flip = 1000 ms/30 ms/60°, field of view = 340 × 340 mm, matrix = 128 × 64, slice thickness = 10 mm, and temporal resolution = 1.0 seconds. Cortical and medullary blood flow values were calculated.
Results
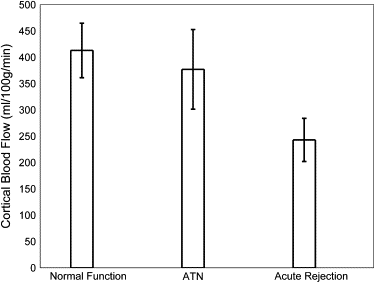
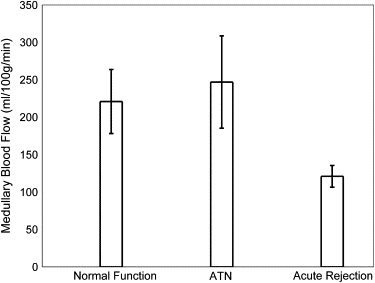
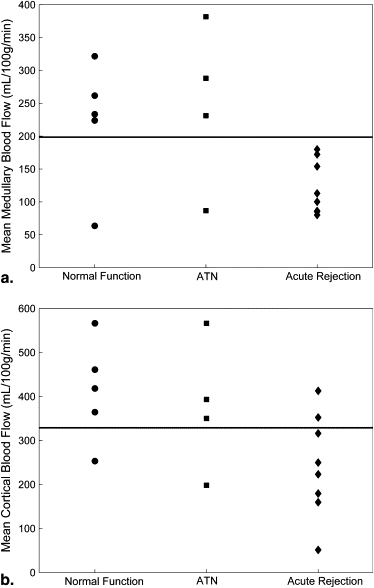
Medullary blood flow values were significantly ( P = .02) lower in allografts undergoing acute rejection (121 ± 41 mL/100 g/min) compared to normal-functioning allografts (221 ± 96 mL/100 g/min) and those with ATN (247 ± 124 mL/100 g/min). Cortical blood flow values were also significantly ( P = .03) reduced in allografts with acute rejection (243 ± 116 mL/100 g/min) compared to those with normal function (413 ± 116 mL/100 g/min).
Conclusions
Preliminary results indicate that MRI perfusion techniques may provide a means of determining noninvasively the viability of renal allografts, potentially alleviating the need for biopsy in some patients.
Kidney transplantation has become the preferred method of kidney replacement therapy since the first successful transplant more than 50 years ago . Although advances in surgical techniques and immunosuppressive therapy have resulted in 1-year graft survival rates exceeding 90%, graft dysfunction in the early posttransplant period remains a serious clinical problem and an important factor in determining the ultimate fate of the allograft . Graft dysfunction in the early posttransplant period results from a variety of causes, including cyclosporine toxicity, infection, vascular compromise, ureteral obstruction, acute tubular necrosis (ATN), and acute rejection . The compounding effects of acute rejection plus delayed graft function (defined as the need for dialysis during the first week posttransplantation) in the transplant course are devastating. Ojo et al. noted that individuals with acute rejection and delayed graft function had a 5-year graft survival of only 35% . Thus, early dysfunction can dramatically influence long-term graft outcomes.
Clinically, a distinction can be made between many of the causes of allograft dysfunction (eg, cyclosporine toxicity can be distinguished from infectious causes on the basis of clinical assessment and laboratory testing). Also, magnetic resonance imaging (MRI) or ultrasound can be used to determine if vascular compromise and ureteral obstruction are present. However, it is difficult to differentiate ATN from acute rejection, because both have similar radiographic and laboratory findings. Biopsy is the only means of differentiating between these two types of dysfunction, yet it has the disadvantages of potential complications and sampling error.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Subjects
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Subject Demographics, Serum Markers, and Blood Flow Values Measured by MRI for Subjects with Normal-Functioning Transplanted Kidneys and Transplanted Kidneys Undergoing ATN or Acute Rejection
Kidney Transplants Normal Functioning ATN Rejection_n_ 5 4 8 Deceased donor 4 4 7 Living related donor 1 0 2 Age (y) 43 ± 10 49 ± 11 49 ± 12 Creatinine (mg/dL) 1.6 ± 0.4 3.5 ± 2.0 4.1 ± 2.0 Hematocrit (%) 36 ± 6 32 ± 3 31 ± 6 Mean medullary blood flow (mL/100 g/min) 221 ± 96 247 ± 124 121 ± 41 Mean cortical blood flow (mL/100 g/min) 413 ± 116 377 ± 152 243 ± 116
ATN: acute tubular necrosis; MRI, magnetic resonance imaging.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
MRI Perfusion Technique
Get Radiology Tree app to read full this article<
MRI Perfusion Calculations
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 2
Pearson Correlation Coefficients, and P Values if Significant, for Serum Markers and MRI-Measured Blood Flow for 17 Renal Transplant Subjects
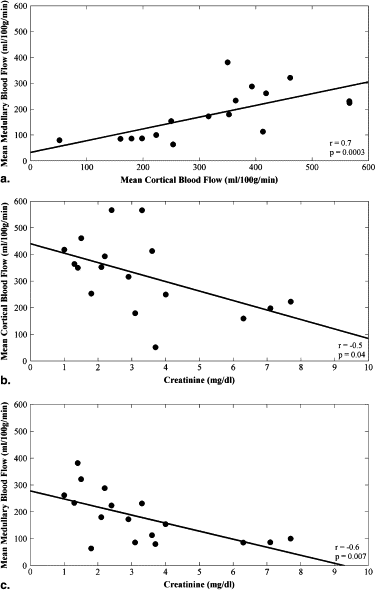
Creatinine (mg/dL) Hematocrit (%) Mean Medullary Blood Flow (mL/100 g/min) Mean Cortical Blood Flow (mL/100 g/min) Creatinine (mg/dL)r = 1.0r = −0.1r = −0.6; P = .007r = −0.5; P = .04 Hematocrit (%)r = 1.0r = 0.1r = 0.2 Mean medullary blood flow (mL/100 g/min)r = 1.0r = 0.7; P = .003 Mean cortical blood flow (mL/100 g/min)r = 1.0
MRI, magnetic resonance imaging.
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Appendix
Theory
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Cm(t)=−kTEln(S(t)So), C
m
(
t
)
=
−
k
TE
ln
(
S
(
t
)
S
o
)
,
where S 0 is the signal intensity prior to the arrival of contrast material, TE is the echo time of the MR imaging sequence, and k is dependent on the R2∗ relaxivity of the contrast agent. C m (t) was calculated according to Eq. (1) . C m (t) was defined for both a major artery (renal or aorta), C a (t), as well as the tissue of the transplanted kidney, C tiss (t). C a (t) is also referred to as the AIF.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
[Ctiss(t)]={[H(t)]⊗[Ca(t)]}. [
C
tiss
(
t
)
]
=
{
[
H
(
t
)
]
⊗
[
C
a
(
t
)
]
}
.
For this study, C tiss (t) was fit by the iterative method described below. An initial guess, H 0 (t), for H(t) was made using the lag-normal model (46):
H(t)=2Cσπ√∫t−tlag0e−(−tc)2/σ2∗e−(t−tlag−)/γd H
(
t
)
=
2
C
σ
π
∫
0
t
−
t
lag
e
−
(
−
t
c
)
2
/
σ
2
∗
e
−
(
t
−
t
lag
−
)
/
γ
d
where C , t c , σ, γ, and t lag are free variables. C a (t) was then convolved with H 0 (t) to yield C ‘ tiss (t), where the prime indicates that this is an intermediate approximation. A chi-squared quality of fit value was calculated for C’ tiss (t) with respect to the measured tissue data, C m (t) ( Figure 1 c). The five parameters defining H 0 (t) were then systematically modified, to produce H ‘ (t), where again the prime indicates an intermediate approximation. The process was iterated until a minimum for the chi-squared value was found using a Nelder-Mead simplex search algorithm. The H’(t), and C’ tiss (t) associated with the minimum chi-squared value were defined as the final H(t) and C tiss (t) ( Fig. 1 c).
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
MTT=∫H(t)dtHmax, MTT
=
∫
H
(
t
)
dt
H
max
,
where H max denotes the maximum value of H(t). The regional renal blood volume (rRBV) was calculated from C tiss (t) and C a (t) by:
rRBV=κ∫0tCtiss(t)dtρ∫0tCa(t)dt, rRBV
=
κ
∫
0
t
C
tiss
(
t
)
dt
ρ
∫
0
t
C
a
(
t
)
dt
,
where κ is a function of the measured hematocrit (taken to equal 0.73 in the present work) and ρ is the density of the kidney (taken to equal 1.04 g/mL in the present work). Finally, in accordance with the central volume principle, the quantitative renal blood flow was calculated by:
RBF=rRBVMTT. RBF
=
rRBV
MTT
.
Get Radiology Tree app to read full this article<
References
1. Sayegh M.H., Carpenter C.B.: Transplantation 50 years later—progress, challenges, and promises. N Engl J Med 2004; 351: pp. 2761-2766.
2. Pascual M., Theruvath T., Kawai T., et. al.: Strategies to improve long-term outcomes after renal transplantation. N Engl J Med 2002; 346: pp. 580-590.
3. Ojo A.O., Wolfe R.A., Held P.J., et. al.: Delayed graft function: risk factors and implications for renal allograft survival. Transplantation 1997; 63: pp. 968-974.
4. Annual reports from the scientific registry of transplant recipients. 2007. Available at: http://www.ustransplant.org/annual_reports/current/ . Accessed June 30, 2008.
5. Breza J., Navratil P.: Renal transplantation in adults. BJU Int 1999; 84: pp. 216-223.
6. Wentland A.L., Korosec F.R., Vigen K.K., et. al.: Cine flow measurements using phase contrast with undersampled projections: in vitro validation and preliminary results in vivo. J Magn Reson Imaging 2006; 24: pp. 945-951.
7. Rusinek H., Kaur M., Lee V.S.: Renal magnetic resonance imaging. Curr Opin Nephrol Hypertens 2004; 13: pp. 667-673.
8. Huang A.J., Lee V.S., Rusinek H.: Functional renal MR imaging. Magn Reson Imaging Clin N Am 2004; 12: pp. 469-486. vi
9. Prasad P.V.: Functional MRI of the kidney: tools for translational studies of pathophysiology of renal disease. Am J Physiol Renal Physiol 2006; 290: pp. F958-F974.
10. Sadowski E.A., Fain S.B., Alford S.K., et. al.: Assessment of acute renal transplant rejection with blood oxygen level-dependent MR imaging: initial experience. Radiology 2005; 236: pp. 911-919.
11. Sommer G., Noorbehesht B., Pelc N., et. al.: Normal renal blood flow measurement using phase-contrast cine magnetic resonance imaging. Invest Radiol 1992; 27: pp. 465-470.
12. Wolf R.L., King B.F., Torres V.E., et. al.: Measurement of normal renal artery blood flow: cine phase-contrast MR imaging vs clearance of p-aminohippurate. AJR Am J Roentgenol 1993; 161: pp. 995-1002.
13. Debatin J.F., Ting R.H., Wegmuller H., et. al.: Renal artery blood flow: quantitation with phase-contrast MR imaging with and without breath holding. Radiology 1994; 190: pp. 371-378.
14. Schoenberg S.O., Just A., Bock M., et. al.: Noninvasive analysis of renal artery blood flow dynamics with MR cine phase-contrast flow measurements. Am J Physiol 1997; 272: pp. H2477-H2484.
15. Dujardin M., Sourbron S., Luypaert R., et. al.: Quantification of renal perfusion and function on a voxel-by-voxel basis: a feasibility study. Magn Reson Med 2005; 54: pp. 841-849.
16. Michaely H.J., Kramer H., Oesingmann N., et. al.: Semiquantitative assessment of first-pass renal perfusion at 1.5 T: comparison of 2d saturation recovery sequences with and without parallel imaging. AJR Am J Roentgenol 2007; 188: pp. 919-926.
17. Wang J.J., Hendrich K.S., Jackson E.K., et. al.: Perfusion quantitation in transplanted rat kidney by MRI with arterial spin labeling. Kidney Int 1998; 53: pp. 1783-1791.
18. Dupas B., Bach-Gansmo T., Blancho G., et. al.: Gadolinium-enhanced mr imaging of normal renal transplants. An evaluation of a T1-weighted dynamic echo-planar sequence. Acta Radiol 1999; 40: pp. 250-254.
19. Beckmann N., Joergensen J., Bruttel K., et. al.: Magnetic resonance imaging for the evaluation of rejection of a kidney allograft in the rat. Transpl Int 1996; 9: pp. 175-183.
20. Szolar D.H., Preidler K., Ebner F., et. al.: Functional magnetic resonance imaging of human renal allografts during the post-transplant period: preliminary observations. Magn Reson Imaging 1997; 15: pp. 727-735.
21. Agildere A.M., Tarhan N.C., Bozdagi G., et. al.: Correlation of quantitative dynamic magnetic resonance imaging findings with pathology results in renal transplants: a preliminary report. Transplant Proc 1999; 31: pp. 3312-3316.
22. Sharma R.K., Gupta R.K., Poptani H., et. al.: The magnetic resonance renogram in renal transplant evaluation using dynamic contrast-enhanced MR imaging. Transplantation 1995; 59: pp. 1405-1409.
23. Pallone T.L., Zhang Z., Rhinehart K.: Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol 2003; 284: pp. F253-F266.
24. Norman J.T., Stidwill R., Singer M., et. al.: Angiotensin II blockade augments renal cortical microvascular pO 2 indicating a novel, potentially renoprotective action. Nephron Physiol 2003; 94: pp. 39-46.
25. Just A., Arendshorst W.J.: Dynamics and contribution of mechanisms mediating renal blood flow autoregulation. Am J Physiol Regul Integr Comp Physiol 2003; 285: pp. R619-R631.
26. Martirosian P., Klose U., Mader I., et. al.: FAIR true-FISP perfusion imaging of the kidneys. Magn Reson Med 2004; 51: pp. 353-361.
27. Schurek H.J., Johns O.: Is tubuloglomerular feedback a tool to prevent nephron oxygen deficiency?. Kidney Int 1997; 51: pp. 386-392.
28. Brezis M., Agmon Y., Epstein F.H.: Determinants of intrarenal oxygenation. I. Effects of diuretics. Am J Physiol 1994; 267: pp. F1059-F1062.
29. Brezis M., Heyman S.N., Epstein F.H.: Determinants of intrarenal oxygenation. II. Hemodynamic effects. Am J Physiol 1994; 267: pp. F1063-F1068.
30. Bonventre J.V., Weinberg J.M.: Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 2003; 14: pp. 2199-2210.
31. Racusen L.C., Halloran P.F., Solez K.: Banff 2003 meeting report: new diagnostic insights and standards. Am J Transplant 2004; 4: pp. 1562-1566.
32. Jani A., Polhemus C., Corrigan G., et. al.: Determinants of hypofiltration during acute renal allograft rejection. J Am Soc Nephrol 2002; 13: pp. 773-778.
33. Zhang J.L., Rusinek H., Bokacheva L., et. al.: Functional assessment of the kidney from magnetic resonance and computed tomography renography: impulse retention approach to a multicompartment model. Magn Reson Med 2008; 59: pp. 278-288.
34. Marckmann P., Skov L., Rossen K., et. al.: Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 2006; 17: pp. 2359-2362.
35. Sadowski E.A., Bennett L.K., Chan M.R., et. al.: Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 2007; 243: pp. 148-157.
36. Thomsen H.S., Marckmann P., Logager V.B.: Enhanced computed tomography or magnetic resonance imaging: a choice between contrast medium-induced nephropathy and nephrogenic systemic fibrosis?. Acta Radiol 2007; 48: pp. 593-596.
37. Sieber M.A., Lengsfeld P., Frenzel T., et. al.: Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol 2008; 18: pp. 2164-2173.
38. Zeirler K.: Theoretical basis of indicator-dilution methods for measuring flow and volume. Circ Res 1962; 10: pp. 393-407.
39. Bassingthwaighte J.B.: Physiology and theory of tracer washout techniques for the estimation of myocardial blood flow: flow estimation from tracer washout. Prog Cardiovasc Dis 1977; 20: pp. 165-189.
40. Montet X., Ivancevic M.K., Belenger J., et. al.: Noninvasive measurement of absolute renal perfusion by contrast medium-enhanced magnetic resonance imaging. Invest Radiol 2003; 38: pp. 584-592.
41. Smith A.M., Grandin C.B., Duprez T., et. al.: Whole brain quantitative CBF and CBV measurements using MRI bolus tracking: comparison of methodologies. Magn Reson Med 2000; 43: pp. 559-564.
42. Rempp K.A., Brix G., Wenz F., et. al.: Quantification of regional cerebral blood flow and volume with dynamic susceptibility contrast-enhanced MR imaging. Radiology 1994; 193: pp. 637-641.
43. Hermoye L., Annet L., Lemmerling P., et. al.: Calculation of the renal perfusion and glomerular filtration rate from the renal impulse response obtained with MRI. Magn Reson Med 2004; 51: pp. 1017-1025.
44. Johnson G., Wetzel S.G., Cha S., et. al.: Measuring blood volume and vascular transfer constant from dynamic, T2∗-weighted contrast-enhanced MRI. Magn Reson Med 2004; 51: pp. 961-968.
45. Ostergaard L., Weisskoff R.M., Chesler D.A., et. al.: High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: mathematical approach and statistical analysis. Magn Reson Med 1996; 36: pp. 715-725.
![Figure 1, (a) Gradient-echo single shot echo-planar image (repetition time [TR]/echo time [TE]/flip = 1000 ms/30 ms/60°, 128 × 64 acquisition matrix, 34 × 34 cm acquisition field of view [FOV]) illustrating region of interest (ROI) placement over the medulla (lower signal regions; lower arrow ) and cortex (higher signal regions; upper arrow ) of a normal-functioning allograft. (b) T1-weighted gradient-echo image of the same region for reference (TR/TE/flip = 87 ms/8 ms/40°, 256 × 256 acquisition matrix, 34 × 34 cm acquisition FOV). The left iliac vein and portion of the abdominal aorta are also seen. Measured concentration versus time data, C m (t) (C; dotted distribution ), and fit curves, C tiss (t) (C; solid line ), associated with the cortical ROI.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/QuantitativeMRMeasuresofIntrarenalPerfusionintheAssessmentofTransplantedKidneys/0_1s20S1076633209002554.jpg)