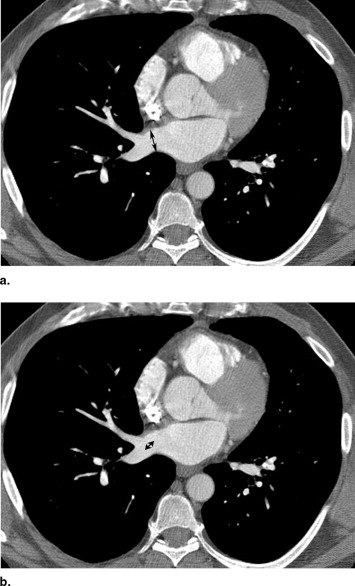
Rationale and Objectives
To evaluate the interobserver agreement of readers in evaluating pulmonary venous anatomy and in measuring pulmonary vein ostial diameters and distance to first bifurcation.
Materials and Methods
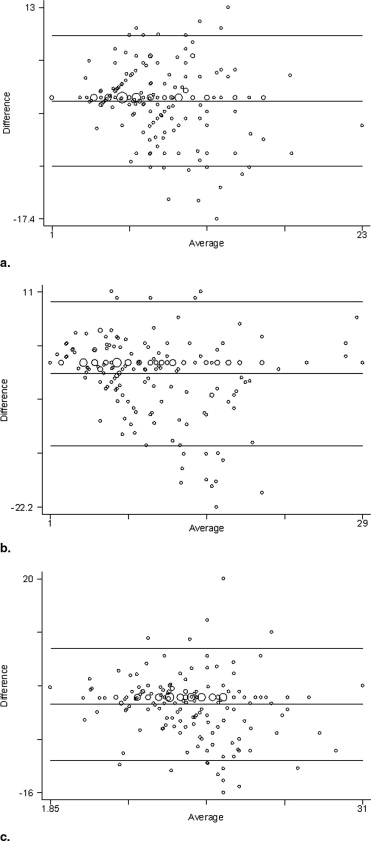
This study was approved by our institutional review board. Thin-section contrast material–enhanced multidetector computed tomography examinations of the thorax were retrospectively reviewed in 200 consecutive patients (38 females and 162 males), age 24–79 years (mean, 52.8) referred for imaging before radiofrequency ablation therapy for atrial fibrillation. For each patient, pulmonary venous anatomy and drainage patterns including the number of venous ostia was assessed independently by experienced cardiothoracic radiologists. Pulmonary vein ostial diameter and distance to the first bifurcation of the four major pulmonary veins (right inferior and superior, left inferior and superior), the middle lobe pulmonary vein, and any anomalous pulmonary veins (common trunks and accessory veins) were measured independently at a workstation. Interreader assessment of pulmonary venous anatomy was evaluated using the Kappa statistic. Interreader variation in measurements of venous diameter and distant to first bifurcation were estimated by Bland-Altman plots and Pitman’s test of difference in variance.
Results
Very good to excellent interreader agreement in detection of anomalous pulmonary venous anatomy, middle lobe pulmonary venous drainage, and other thoracic venous anomalies. No significant variation between readers in pulmonary vein ostial diameter measurements for the four major and middle lobe pulmonary veins, or the anomalous pulmonary veins. Significant interreader variability was noted in measurements of the pulmonary vein distance to first bifurcation for the right inferior ( P = .017), middle lobe ( P = .005), and left inferior ( P = .015) pulmonary veins.
Conclusions
There is excellent interobserver agreement when evaluating normal and anomalous pulmonary venous drainage patterns, and when measuring normal or anomalous pulmonary vein diameters. However, measurements of distances to first bifurcation were less reliable across readers.
Atrial fibrillation is the most commonly sustained cardiac arrhythmia, and its prevalence increases with age. It is a major cause of stroke and is associated with significant morbidity and mortality. Atrial ectopic beats within the pulmonary veins have been shown to initiate atrial fibrillation in most patients. The importance of the pulmonary veins pulmonary veins in the initiation of atrial fibrillation (AF) was first demonstrated by Haissaguerre et al ( ). They also demonstrated that pulmonary vein isolation could eliminate this arrhythmia, which represented a major therapeutic advance. Numerous studies have demonstrated the role of the posterior left atrium as well as the pulmonary veins in atrial fibrillation ( ). Subsequently, various catheter-based pulmonary vein ablation techniques, such as radiofrequency ablation and cryoablation, have been developed and used clinically, to electrically confine pulmonary veins triggers ( ). Two ablation strategies have been developed: segmental ostial isolation ( ) and circular extraostial isolation ( ), each with its own advantages and disadvantages ( ).
Many imaging modalities are employed to delineate the anatomy of the pulmonary veins and the left atrium, both pre- and postablation. These include computed tomography (CT) ( ), three-dimensional (3D) magnetic resonance angiography (MRA) ( ), pulmonary vein angiography ( ), and intracardiac ultrasound ( ). CT has the advantage of being fast, widely available, easy for patients to tolerate, and has a high spatial resolution, but has the disadvantage of using ionizing radiation. MRA has the advantage of being free of ionizing radiation, but it also has been shown to be particularly effective in providing detailed and complete imaging of the anatomy of the pulmonary veins and left atrium ( ), but has several disadvantages, including many atrial fibrillation patients have pacemakers or defibrillators and therefore have a contraindication to MRA. Also, magnetic resonance (MR) imaging cannot be performed in patients with claustrophobia or in patients who, because of their clinical conditions, cannot tolerate the considerably long imaging times of MR imaging ( ). Pulmonary vein venography also has the disadvantage of using ionizing radiation and the fluoroscopy times of these procedures are substantial, in part because of the laborious visualization of the pulmonary veins and can not adequately depict this anatomy ( ).
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Methods and materials
Subjects
Get Radiology Tree app to read full this article<
Imaging
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Pulmonary Vein Anatomy
Get Radiology Tree app to read full this article<
Table 1a
Absence or Presence of Normal Pulmonary Veins (Kappa Statistic)
Absence or Presence of Normal Pulmonary Vein Readers RSPV RIPV MLPV LSPV LIPV MLPV Drainage Pattern First vs. second reader 1.0 1.0 1.0 1.0 1.0 0.88 (0.82–0.94)
RSPV: right superior pulmonary vein; RIPV: right inferior pulmonary vein; MLPV: middle lobe pulmonary vein; LSPV: left superior pulmonary vein; LIPV: left inferior pulmonary vein.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1b
Absence or Presence of Variant Pulmonary Veins (Kappa Statistic)
Absence or Presence of Variant Pulmonary vein Readers CRT CLT ARPV ALPV OAPV First vs. second reader 1.0 0.84 (0.56–1.0) 0.92 (0.75–1.0) 1.0 1.0
CRT: common right trunk; CLT: common left trunk; ARPV: accessory right pulmonary vein; ALPV: accessory left pulmonary vein; OAPV: other accessory pulmonary vein.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Pulmonary Vein Diameter
Get Radiology Tree app to read full this article<
Table 2a
Interobserver Variability of Normal Pulmonary Vein Diameters (Bland-Altman Plots and Pitman Coefficients)
Pulmonary Vein Pulmonary Vein Diameter (in mm) Pitman’s Test of Difference in Variance ( r ) Reader 1 (SD) Reader 2 (SD) Mean Difference Right superior 17.9 (4.6) 16.9 (4.2) 2.0 (2.5) 0.12 Right inferior 16.9 (3.5) 16.7 (3.8) 1.8 (2.5) −0.10 Right middle 8.6 (2.3) 8.3 (2.4) 1.3 (1.5) −0.09 Left superior 16.4 (3.6) 15.9 (3.7) 1.8 (2.2) −0.08 Left inferior 14.2 (3.6) 14.7 (3.6) 2.1 (2.5) 0.01
RSPV: right superior pulmonary vein; RIPV: right inferior pulmonary vein; MLPV: middle lobe pulmonary vein; LSPV: left superior pulmonary vein; LIPV: left inferior pulmonary vein.
*Statistically significant difference, P < .05.
Table 2b
Interobserver Variability of Anomalous Pulmonary Vein Diameters (Bland-Altman Plots and Pitman Coefficients)
Pulmonary Vein Pulmonary Vein Diameter (in mm) Pitman’s test of Difference in Variance ( r ) Reader 1 (SD) Reader 2 (SD) Mean Difference Common right trunk 35.3 (11.7) 35.0 (14.1) ‡ Common left trunk 21.8 (5.4) 25.9 (4.1) 0.13 Accessory right 8.4 (1.2) 7.7 (1.8) −0.75
CRT: common right trunk; CLT: common left trunk; ARPV: accessory right pulmonary vein.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Distance to First Bifurcation
Get Radiology Tree app to read full this article<
Table 3a
Interobserver Variability of Normal Pulmonary Vein Distance to First Bifurcation (Bland-Altman Plots and Pitman Coefficients)
Pulmonary Vein Pulmonary Vein Distance to First Bifurcation (in mm) Pitman’s test of difference in variance ( r ) Reader 1 (SD) Reader 2 (SD) Mean Difference Right superior 15.6 (6.4) 16.4 (6.7) 3.3 (3.9) −0.08 Right inferior 8.2 (3.7) 8.6 (4.4) 2.9 (3.6) −0.17 ⁎ Right middle 9.2 (5.6) 10.8 (6.6) 3.4 (4.6) −0.21 ⁎ Left superior 18.4 (6.8) 18.3 (6.7) 3.4 (4.4) 0.01 Left inferior 14.1 (4.7) 15.2 (5.4) 2.9 (3.9) −0.18 ⁎
RSPV: right superior pulmonary vein; RIPV: right inferior pulmonary vein; MLPV: middle lobe pulmonary vein; LSPV: left superior pulmonary vein; LIPV: left inferior pulmonary vein.
Get Radiology Tree app to read full this article<
Table 3b
Interobserver Variability of Anomalous Pulmonary Vein Distance to First Bifurcation (Bland-Altman Plots and Pitman Coefficients)
Pulmonary Vein Pulmonary Vein Distance to First Bifurcation (in mm) Pitman’s test of Difference in Variance ( r ) Reader 1 (SD) Reader 2 (SD) Mean Difference Common right trunk 9.0 (11.2) 13.5 (2.1) ‡ Common left trunk 10.7 (7.0) 11.9 (7.7) −0.35 Accessory right 10.0 (4.7) 8.6 (5.9) −0.35
CRT: common right trunk; CLT: common left trunk; ARPV: accessory right pulmonary vein.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Limitations
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Haïssaguerre M., Jaïs P., Shah D.C., et. al.: Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339: pp. 659-666.
2. Wu T., Ong J., Chang C.M., et. al.: Pulmonary veins and ligament of Marshall as sources of rapid activations in a canine model of sustained atrial fibrillation. Circulation 2001; 103: pp. 1157-1163.
3. Skanes A., Mandapati R., Berenfeld O., et. al.: Spatiotemporal periodicity during atrial fibrillation in the isolated sheep heart. Circulation 2001; 98: pp. 1236-1248.
4. Mandapati R., Skanes A.C., Chen J., et. al.: Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation 2000; 101: pp. 194-199.
5. Yuan X.P., Bach D., Skanes A., et. al.: Assessment of intra- and interobserver variability of pulmonary vein measurements from CT angiography. Acad Radiol 2004; 11: pp. 1211-1218.
6. Marrouche N.F., Dresing T., Cole C., et. al.: Circular mapping and ablation of the pulmonary vein for treatment of atrial fibrillation impact of different catheter technologies. J Am Coll Cardiol 2002; 40: pp. 464-474.
7. Tse H.F., Reek S., Timmermans C., et. al.: Pulmonary vein isolation using transvenous catheter cryoablation for treatment of atrial fibrillation without risk of pulmonary vein stenosis. J Am Coll Cardiol 2003; 42: pp. 752-758.
8. Marrouche N.F., Cole C., Pavia S., et. al.: Pulmonary veins isolation for treatment of atrial fibrillation different catheter technologies for pulmonary veins isolation. Circulation 2001; 104: pp. II-620.
9. Haïssaguerre M., Shah D., Jaïs P., et. al.: Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation 2000; 102: pp. 2463-2465.
10. Pappone C., Rosanio S., Oreto G., et. al.: Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation 2000; 102: pp. 2619-2628.
11. Mansour M., Holmvang G., Sosnovik D., et. al.: Assessment of pulmonary vein anatomic variability by magnetic resonance imaging: implications for catheter ablation techniques for atrial fibrillation. J Cardiovasc Electrophysiol 2004; 15: pp. 387-393.
12. Scharf C., Sneider M., Case I., et. al.: Anatomy of the pulmonary veins in patients with atrial fibrillation and effects of segmental ostial ablation analyzed by computed tomography. J Cardiovasc Electrophysiol 2003; 14: pp. 150-155.
13. Kato R., Lickfett L., Meininger G., et. al.: Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation lessons learned by use of magnetic resonance imaging. Circulation 2003; 107: pp. 2004.
14. Wittkampf F.H., Vonken E.J., Derksen R., et. al.: Pulmonary vein ostium geometry: analysis by magnetic resonance angiography. Circulation 2003; 107: pp. 21-23.
15. Tsao H., Yu W., Cheng H., et. al.: Pulmonary vein dilatation in patients with atrial fibrillation: Detection by magnetic resonance imaging. J Cardiovasc Electrophysiol 2001; 12: pp. 809-813.
16. Lin W., Prakash V., Tai C., et. al.: Pulmonary vein morphology in patients with paroxysmal atrial fibrillation initiated by ectopic beats originating from the pulmonary veins implications for catheter ablation. Circulation 2000; 101: pp. 1274-1281.
17. Cabrera J.A., Sánchez-Quintana D., Farré J., et. al.: Ultrasonic characterization of the pulmonary venous wall. Circulation 2002; 106: pp. 968-973.
18. Saad E.B., Cole C.R., Marrouche N.F., et. al.: Use of intracardiac echocardiography for prediction of chronic pulmonary vein stenosis after ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2002; 13: pp. 986-989.
19. Lacomis J.M., Wigginton W., Fuhrman C., et. al.: Multi-detector row CT of the left atrium and pulmonary veins before radio-frequency catheter ablation for atrial fibrillation. Radiographics 2003; 23: pp. S35-S48.
20. Cronin P., Sneider M.B., Kazerooni E.A., et. al.: MDCT of the left atrium and pulmonary veins in planning radiofrequency ablation for atrial fibrillation: a how-to guide. AJR Am J Roentgenol 2004; 183: pp. 767-778.
21. Taylor G.W., Kay G.N., Zheng X., et. al.: Pathological effects of extensive radiofrequency energy applications in the pulmonary veins in dogs. Circulation 2000; 101: pp. 1736-1742.
22. Saad E.B., Marrouche N.F., Saad C.P., et. al.: Pulmonary vein stenosis after catheter ablation of atrial fibrillation emergence of a new clinical syndrome. Ann Intern Med 2003; 138: pp. 634-638.
23. Yu W.C., Hsu T.L., Tai C.T., et. al.: Acquired pulmonary vein stenosis after radiofrequency catheter ablation of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2001; 12: pp. 887-892.
24. Jongbloed M.R., Bax J.J., de Groot N.M., et. al.: Radiofrequency catheter ablation of paroxysmal atrial fibrillation; guidance by intracardiac echocardiography and integration with other imaging techniques. Eur J Echocardiogr 2003; 4: pp. 54-58.
25. Schwartzman D., Lacomis J., Wigginton W.G.: Characterization of left atrium and distal pulmonary vein morphology using multidimensional computed tomography. J Am Coll Cardiol 2003; 41: pp. 1349-1357.
26. Wood M.A., Wittkamp M., Henry D., et. al.: A comparison of pulmonary vein ostial anatomy by computerized tomography, echocardiography, and venography in patients with atrial fibrillation having radiofrequency catheter ablation. Am J Cardiol 2004; 93: pp. 49-53.
27. Bland J.M., Altman D.G.: Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: pp. 307-310.
28. Hendel R.C., Patel M.R., Kramer C.M., et. al., American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology: ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol 2006; 48: pp. 1475-1497.
29. Kim Y.H., Marom E.M., Herndon J.E., et. al.: Pulmonary vein diameter, cross-sectional area, and shape: CT analysis. Radiology 2005; 235: pp. 43-49.
30. Cronin P., Kelly A.M., Desjardins B., et. al.: Normative analysis of pulmonary vein drainage patterns on multidetector CT with measurements of pulmonary vein ostial diameters and distance to first bifurcation. Acad Radiol 2007; 14: pp. 178-188.
31. Yuan X.P., Bach D., Skanes A., et. al.: Assessment of intra- and interobserver variability of pulmonary vein measurements from CT angiography. Acad Radiol 2004; 11: pp. 1211-1218.