Rationale and Objectives
In patients with adenomyosis, the severity of symptoms correlates roughly with the extent of adenomyosis. Thus, it was hypothesized that the ablation of enough volume of adenomyosis might alleviate symptoms. The aim of this study was to investigate the safety and efficacy of high-intensity focused ultrasound (HIFU) ablation for the treatment of adenomyosis.
Materials and Methods
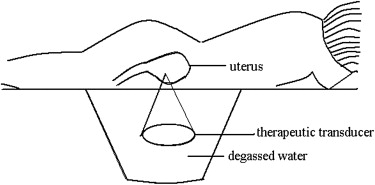
Phase I HIFU ablation of adenomyosis was performed on 12 patients. Three patients each were treated using four different acoustic intensities (290, 340, 380, and 420 W) step by step. Contrast-enhanced ultrasound was used to evaluate the necrotic region of treated adenomyosis. The efficacy of therapy was evaluated after 3 months of follow-up.
Results
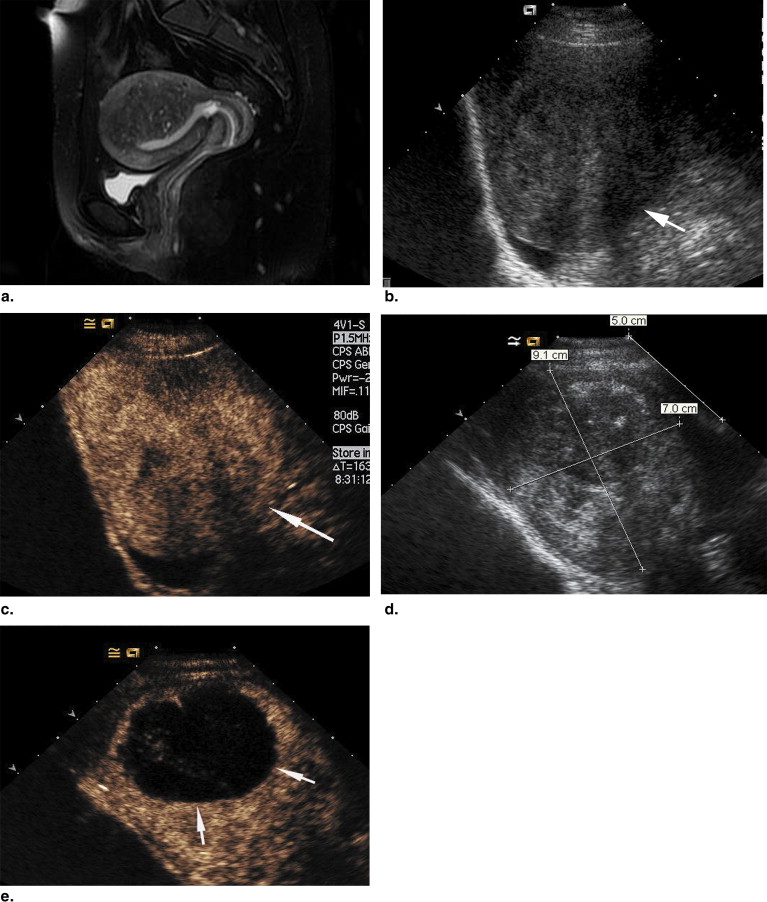
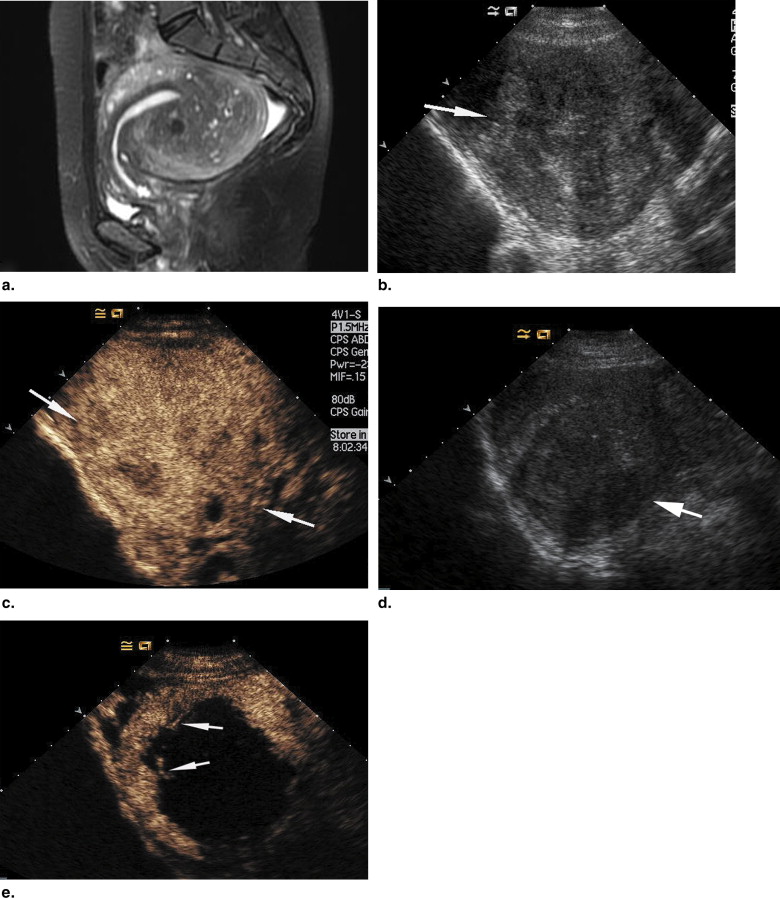
All patients in the four groups tolerated the therapy well, and no severe complications were found during follow-up. After treatment, nonenhanced necrotic regions were shown on contrast-enhanced ultrasound in all treated adenomyosis. The mean volumes of the nonenhanced regions were 72, 75, 68, and 124 cm 3 in the 290-W, 340-W, 380-W, and 420-W groups, respectively. At 3 months after therapy, the mean pain relief in the four groups was 25%, 58.3%, 66.7%, and 83.3%, respectively.
Conclusions
HIFU may be a safe and effective method to treat adenomyosis, and an acoustic intensity of 420 W may be able to produce larger volumes of necrosis and better pain relief.
Adenomyosis is a common gynecologic disorder that affects women during their reproductive lives. It is characterized by the presence of ectopic endometrial glands and stroma in the myometrium . About two thirds of women are symptomatic with menorrhagia and dysmenorrhea. The usual treatment for symptomatic women is hysterectomy, which may be performed in women who are not intent on preserving their uteruses . However, hysterectomy cannot be performed in patients who either have not completed their families or do not wish to lose their uteruses. Conservative surgery such as the local excision of adenomyotic lesions, while preserving fertility, is difficult because of the ill-defined endometrial-myometrial boundaries . Surgery may also result in fibrotic scars and sutures in surrounding healthy tissue, which can negatively affect future fertility. Hysteroscopic resection may be performed in some women with superficial adenomyosis . Deep adenomyotic lesions cannot be removed using this technique, and residual adenomyosis-related symptoms may remain after therapy. Symptoms may reappear after the discontinuation of drug administration in medical therapies . Thus, a new, effective method for the treatment of patients with adenomyosis would be of great value.
High-intensity focused ultrasound (HIFU) ablation has been used to treat patients with a variety of malignancies using an external ultrasound energy source to induce thermal ablation of tumors at depth, through the intact skin . In contrast to other energy sources that have been used to induce coagulation necrosis of target tumors in clinical practice, such as radiofrequency, microwave, cryotherapy, and laser, HIFU does not require the insertion of an applicator into the target tissue, and thus, there is no risk for puncture-related bleeding and seeding of metastases, and the procedure may be performed in patients with poor blood coagulability. The volume of the necrotic region is not limited by the technique itself, and thus, large-volume or irregular lesions can be ablated under real-time imaging guidance. HIFU ablation has been used to treat patients with uterine fibroids, and the results have shown decreases in fibroid size and associated symptoms . As for adenomyosis, there have been several case reports of using magnetic resonance–guided focused ultrasound for focal adenomyosis, with satisfactory results . Fukunishi et al reported their early results of magnetic resonance–guided focused ultrasound surgery of adenomyosis in 20 patients. Although these results indicated the safe and effective ablation of adenomyosis tissue using focused ultrasound, further studies are needed for the determination of its final value. Unlike previous studies using HIFU guided by magnetic resonance imaging, in this study, we used real-time ultrasound to target lesions during HIFU therapy, because until now, all HIFU transducers in China have been integrated with diagnostic ultrasound probes, and real-time ultrasound has proved a useful technique for guiding HIFU therapy . Thus, this study was designed to evaluate the safety and efficacy of ultrasound-guided HIFU ablation using different ultrasound intensity levels for the treatment of patients with adenomyosis.
Materials and methods
Patients
Get Radiology Tree app to read full this article<
Equipment
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Preparation and Ultrasound Examination
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Treatment
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Post-treatment Contrast-enhanced Imaging
Get Radiology Tree app to read full this article<
Post-treatment Follow-up
Get Radiology Tree app to read full this article<
Results
Treatment Data
Get Radiology Tree app to read full this article<
Table 1
Treatment Data of High Intensity Focused Ultrasound (HIFU) Ablation Therapy for Adenomyosis
Power (W) Therapy Time (s) Total Power (J) 290 1430 ± 1065 431,267 ± 300,731 340 1132 ± 497 384,637 ± 160,329 380 1637 ± 600 618,895 ± 240,504 420 1820 ± 1475 765,571 ± 618,741
Get Radiology Tree app to read full this article<
Safety of HIFU Ablation and Symptom Relief
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Imaging Assessment of HIFU Ablation
Get Radiology Tree app to read full this article<
Table 2
Contrast-Enhanced Ultrasonography of Uterus After High Intensity Focused Ultrasound (HIFU) Ablation Therapy
Power (W) Uterine Volume (cm 3 ) Necrotic Volume (cm 3 ) Volume Percentage of Nonenhanced to the Whole Uterus (%) 290 226 ± 161 72 ± 75 25 ± 15 340 251 ± 125 75 ± 62 26 ± 11 380 253 ± 142 68 ± 51 30 ± 19 420 213 ± 149 124 ± 121 51 ± 15
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Ota H., Igarashi S., Hatazawa J., et. al.: Is adenomyosis an immune disease?. Hum Reprod Update 1998; 4: pp. 360-367.
2. Byun J.Y., Kim S.E., Choi B.G., et. al.: Diffuse and focal adenomyosis: MR imaging findings. Radiographics 1999; 19: pp. S161-S170.
3. Vavilis D., Agorastos T., Tzafetas J., et. al.: Adenomyosis at hysterectomy: prevalence and relationship to operative findings and reproductive and menstrual factors. Clin Exp Obstet Gynecol 1997; 24: pp. 36-38.
4. Parazzini F., Mais V., Cipriani S., et. al.: Determinants of adenomyosis in women who underwent hysterectomy for benign gynecological conditions: results from a prospective multicentric study in Italy. Eur J Obstet Gynecol Reprod Biol 2009; 143: pp. 103-106.
5. Wood C.: Surgical and medical treatment of adenomyosis. Hum Reprod Update 1998; 4: pp. 323-336.
6. Keckstein J.: Hysteroscopy and adenomyosis. Contrib Gynecol Obstet 2000; 20: pp. 41-50.
7. Grow D.R., Filer R.B.: Treatment of adenomyosis with long-term GnRH analogues: a case report. Obster Gynecol 1991; 78: pp. 538-539.
8. Gianfelice D., Khiat A., Boulanger Y., et. al.: Feasibility of magnetic resonance imaging–guided focused ultrasound surgery as an adjunct to tamoxifen therapy in high-risk surgical patients with breast carcinoma. J Vasc Interv Radiol 2003; 14: pp. 1275-1282.
9. Wu F., Wang Z.B., Chen W.Z., et. al.: Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas: early Chinese clinical experience. Ultrasound Med Biol 2004; 30: pp. 245-260.
10. Klingler H.C., Susani M., Seip R., et. al.: A novel approach to energy ablative therapy of small renal tumours: laparoscopic high-intensity focused ultrasound. Eur Urol 2008; 53: pp. 810-816.
11. Held R.T., Zderic V., Nguyen T.N., et. al.: Annular phased-array high-intensity focused ultrasound device for image-guided therapy of uterine fibroids. IEEE Trans Ultrason Ferroelectr Freq Control 2006; 53: pp. 335-348.
12. Ren X.L., Zhou X.D., Zhang J., et. al.: Extracorporeal ablation of uterine fibroids with high-intensity focused ultrasound: imaging and histopathologic evaluation. J Ultrasound Med 2007; 26: pp. 201-212.
13. Rabinovici J., Inbar Y., Revel A., et. al.: Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol 2007; 30: pp. 771-777.
14. Hesley G.K., Gorny K.R., Henrichsen T.L., et. al.: A clinical review of focused ultrasound ablation with magnetic resonance guidance: an option for treating uterine fibroids. Ultrasound Q 2008; 24: pp. 131-139.
15. Fennessy F.M., Tempany C.M.: MRI-guided focused ultrasound surgery of uterine leiomyomas. Acad Radiol 2005; 12: pp. 1158-1166.
16. Rabinovici J., Inbar Y., Eylon S.C., et. al.: Pregnancy and live birth after focused ultrasound surgery for symptomatic focal adenomyosis: a case report. Hum Reprod 2006; 21: pp. 1255-1259.
17. Yoon S.W., Kim K.A., Cha S.H., et. al.: Successful use of magnetic resonance-guided focused ultrasound surgery to relieve symptoms in a patient with symptomatic focal adenomyosis. Fertil Steril 2008; 90: pp. 2018.e13-2018.e15.
18. Fukunishi H., Funaki K., Sawada K., et. al.: Early results of magnetic resonance-guided focused ultrasound surgery of adenomyosis: analysis of 20 cases. J Minim Invasive Gynecol 2008; 15: pp. 571-579.
19. Siegelman E.S., Van Deerlin V.: Adenomyosis: current concepts and imaging considerations. AJR Am J Roentgenol 1998; 170: pp. 437-441.
20. Ascher S.M., Arnold L.L., Patt R.H., et. al.: Adenomyosis: prospective comparison of MR imaging and transvaginal sonography. Radiology 1994; 190: pp. 803-806.
21. Gorce J.M., Arditi M., Schneider M.: Influence of bubble size distribution on the echogenicity of ultrasound contrast agents: a study of SonoVue. Invest Radiol 2000; 35: pp. 661-671.
22. Bleuzen A., Tranquart F.: Incidental liver lesions: diagnostic value of cadence contrast pulse sequencing (CPS) and SonoVue. Eur Radiol 2004; 14: pp. 53-62.
23. Rahbin N., Siösteen A.K., Elvin A., et. al.: Detection and characterization of focal liver lesions with contrast-enhanced ultrasonography in patients with hepatitis C-induced liver cirrhosis. Acta Radiol 2008; 49: pp. 251-257.
24. Zhou X.D., Ren X.L., Zhang J., et. al.: Therapeutic response assessment of high intensity focused ultrasound therapy for uterine fibroid: utility of contrast-enhanced ultrasonography. Eur J Radiol 2007; 62: pp. 289-294.
25. Lu M.D., Yu X.L., Li A.H., et. al.: Comparison of contrast enhanced ultrasound and contrast enhanced CT or MRI in monitoring percutaneous thermal ablation procedure in patients with hepatocellular carcinoma: a multi-center study in China. Ultrasound Med Biol 2007; 33: pp. 1736-1749.
26. Orsini L.F., Salardi S., Pilu G., et. al.: Pelvic organs in premenarcheal girls: real-time ultrasonography. Radiology 1984; 153: pp. 113-116.
27. Mehlisch D.R., Fulmer R.I.: A crossover comparison of bromfenac sodium, naproxen sodium, and placebo for relief of pain from primary dysmenorrhea. J Womens Health 1997; 6: pp. 83-92.
28. Sharp N.C., Cronin N., Feldberg I., et. al.: Microwaves for menorrhagia: a new fast technique or endometrial ablation. Lancet 1995; 346: pp. 1003-1004.
29. Nishida M.: Relationship between the onset of dysmenorrhea and histologic findings in adenomyosis. Am J Obstet Gynecol 1991; 165: pp. 229-231.
30. Li F., Zhang Q., Du Y., et. al.: Effects of therapeutic dose on temperature rise induced by high intensity focused ultrasound in tissue. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2003; 20: pp. 466-471.