Rationale and Objectives
Analyze factors that influence participation in research studies that use coronary computed tomography (CT) imaging.
Materials and Methods
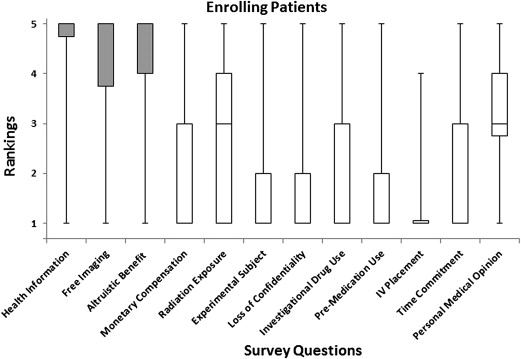
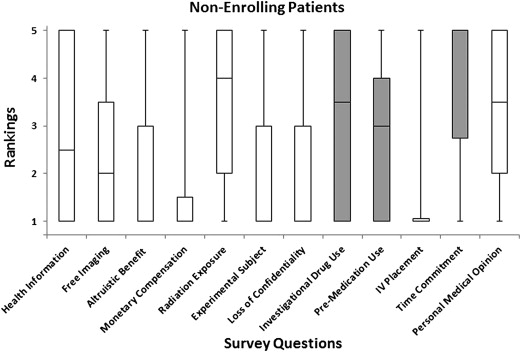
A 12-point survey using a questionnaire was conducted on 80 subjects, of whom 40 agreed to participate in a cardiovascular CT imaging research study (enrolling subjects) and 40 declined participation (non-enrolling subjects). Potential factors that motivated the acceptance or refusal of enrollment were evaluated using a 5-point Likert scale. The following aspects were addressed: (1) additional health information, (2) free imaging, (3) altruistic benefit to society, (4) monetary compensation, (5) radiation exposure, (6) role as an experimental subject, (7) possible loss of confidentiality, (8) contrast or investigational drug use, (9) premedication use, (10) blood draw or intravenous placement, (11) time commitment, and (12) personal medical opinion. Response distributions were obtained for each question and compared between enrolling and non-enrolling groups.
Results
Enrolling subjects gave significantly higher ratings than non-enrolling subjects for the following factors: additional health information ( P < .001), free imaging ( P < .001), and the altruistic benefit to society ( P < .001). For non-enrolling subjects, concern for possible drug use or contrast injection ( P < .001), concern for possible premedication ( P < .001), and personal availability or time commitment ( P < .001) were all given significantly higher ratings. Concern for radiation exposure ( P = .002) and personal medical opinion ( P < .001) received significantly high ratings among both groups but did not differ between groups.
Conclusions
Several influential concerns and benefits were identified from potential research subjects. Knowledge of what influences patient participation in studies involving CT imaging may allow researchers to effectively address concerns and highlight the potential benefits related to participation.
Clinical research is vital for the progress of medicine and clinical practice . Because clinical trials depend on patient participation, effective recruitment strategies are imperative for trial success . Understanding the factors affecting a patient’s willingness to participate in a clinical trial is beneficial for successful patient recruitment because it allows customization of recruitment strategies to address typical concerns and expectations. Inefficiencies in subject recruitment may result in unwanted extensions of study time frames, typically increasing study costs and challenging the completion of expected site deliverables. The investigators’ ability to address common concerns puts research subjects at ease , which enables the trial to avoid participant attrition and helps achieve the expected recruitment goals for the study .
Several studies have examined factors that affect patients’ decisions to participate in different types of clinical trials. The altruistic feeling of helping society has been shown to have a positive influence on participation . The potential to obtain additional health-related information has been shown to affect participation both positively and negatively . Poor education levels and unfamiliarity with scientific jargon typically have been shown to have a negative impact on study participation . Existing studies focus exclusively on the influencing factors on those patients who participate in clinical trials, providing a biased representation of patients’ concerns. To our knowledge, no study has yet analyzed the concerns of patients who decline participation.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
Patient Distribution
Accepted Participation Declined Participation Total Patients contacted to participate in CT clinical trial (male, female) 89 (67, 22) 275 (162, 113) 346 (229, 135) Contacted to participate in questionnaire study (male, female) 55 (37, 13) 79 (36, 43) 134 (78, 56) Participated in questionnaire study (male, female) 40 (20, 20) 40 (18, 22) 80 (38, 42)
CT, computed tomography.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 2
Categories and Questions from Questionnaire
Benefits Risks/Discomforts Possible Side Effects Personal Influence The chance to obtain additional health information Concern regarding possible radiation exposure Concern for possible contrast or investigational drug use Personal availability and/or time commitment The opportunity to obtain free imaging Role as an experimental subject (“guinea pig”) Concern for possible premedication use Personal medical opinion regarding your own health Altruistic benefit to society Concern for possible loss of confidentiality Concern for possible blood draw/intravenous placement Monetary compensation
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 3
Average Response Values for Enrolling and Non-enrolling Subjects
Questions Enrolling Non-enrolling Benefits The chance to obtain additional health information 4.5 ± 1.1 2.7 ± 1.7 The opportunity to obtain free imaging 4.1 ± 1.3 2.4 ± 1.7 Altruistic benefit to society 4.5 ± 0.9 2.8 ± 1.5 Monetary compensation 2.1 ± 1.3 1.7 ± 1.4 Risks/discomforts Concern regarding possible radiation exposure 2.7 ± 1.5 3.6 ± 1.6 Role as an experimental subject (“guinea pig”) 1.7 ± 1.2 2.1 ± 1.3 Concern for possible loss of confidentiality 1.6 ± 1.1 2.1 ± 1.5 Possible side effects Concern for possible contrast or investigational drug use 1.8 ± 1.1 3.1 ± 1.7 Concern for possible premedication use 1.6 ± 1.1 2.9 ± 1.6 Concern for possible blood draw/intravenous placement 1.4 ± 0.9 1.4 ± 0.9 Personal influence Personal availability and/or time commitment 2.0 ± 1.2 3.7 ± 1.6 Personal medical opinion regarding your own health 3.2 ± 1.3 3.3 ± 1.6
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Wendler D., Krohmal B., Emanuel E.J., et. al.: Why patients continue to participate in clinical research. Arch Intern Med 2008; 168: pp. 1294-1299.
2. Heard A., March R., Maguire P., et. al.: Recruitment strategies for an osteoporosis clinical trial: Analysis of effectiveness. Australas J Ageing 2012; 31: pp. 176-180.
3. Mattson M.E., Curb J.D., McArdle R., and the AMIS and BHAT Research Groups: Participation in a clinical trial: The patients’ point of view. Control Clin Trials 1985; 6: pp. 156-167.
4. Albrecht T.L., Penner L.A., Ruckdeschel J.C.: Understanding patient decisions about clinical trials and the associated communication process: A preliminary report. J Cancer Educ 2003; 18: pp. 210-214.
5. Gul R.B., Ali P.A.: Clinical trials: the challenge of recruitment and retention of participants. J Clin Nurs 2009; 19: pp. 227-233.
6. Jenkins V., Fallowfield L.: Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. Br J Cancer 2000; 82: pp. 1783-1788.
7. Ellis P.M., Dowsett S.M., Butow P.N., et. al.: Attitudes to randomized clinical trials amongst out-patients attending a medical oncology clinic. Health Expect 1998; 2: pp. 33-43.
8. Burgess L.J., Sulzer N.U., Hoosain F., et. al.: Patients’ motivations for participating in cardiovascular clinical trials: a local perspective. Cardiovasc J Afr 2009; 20: pp. 220-223.
9. Cassileth B.R., Lusk E.J., Miller D.S., et. al.: Attitudes toward clinical trials among patients and the public. JAMA 1982; 248: pp. 968-970.
10. Verheggen F., Nieman F., Jonkers R.: Determinants of patient participation in clinical studies requiring informed consent: Why patients enter a clinical trial. Patient Educ Couns 1998; 35: pp. 111-125.
11. Slevin M., Mossman J., Bowling A., et. al.: Volunteers or victims: Patients’ views of randomized cancer clinical trials. Br J Cancer 1995; 71: pp. 1270-1274.
12. Meneguin S., Cesar L.A.: Motivation and frustration in cardiology trial participation: The patient perspective. Clinics 2012; 67: pp. 603-607.
13. Canvin K., Jacoby A.: Duty, desire or indifference? A qualitative study of patient decisions about recruitment to an epilepsy treatment trial. Trials 2006; 7: pp. 32.
14. Ludwig R.L., Turner L.W.: Effective patient education in medical imaging: Public perceptions of radiation exposure risk. J Allied Health 2002; 31: pp. 159-164.
15. Haramati L.B.: Ethical trials to determine the risks and benefits of radiation exposure from coronary CT angiography. J Am Coll Radiol 2008; 5: pp. 1073-1076.
16. Budoff M.J., Gupta M.: Radiation exposure from cardiac imaging procedures. J Am Coll Cardiol 2010; 56: pp. 712-714.
17. Berlin L.: Communicating the harmful effects of radiation exposure from medical imaging: malpractice considerations. Health Phys 2011 Nov; 101: pp. 583-588.
18. Shehadi W.H.: Clinical problems and toxicity of contrast agents. Am J Roentgenol Radium Ther Nucl Med 1966; 97: pp. 762-771.
19. Conrad P.: The meaning of medications: Another look at compliance. Soc Sci Med 1985; 20: pp. 29-37.
20. Deber R.B., Kraetschmer N., Irvine J.: What role do patients wish to play in treatment decision making?. Arch Intern Med 1996; 156: pp. 1414-1420.
21. Coulter A., Entwistle V., Gilbert D.: Sharing decisions with patients: is the information good enough?. BMJ 1999; 318: pp. 318-322.
22. Roe K.M., Minkler M., Saunders F.F.: Combining research, advocacy and education: The methods of the grandparent caregiver study. Health Educ Behav 1995; 22: pp. 458-475.
23. Duke University. PROspective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE). In: ClinicalTrials.gov Bethesda, MD: National Library of Medicine (US). 2000- Available at: http://clinicaltrials.gov/show/NCT01174550 . Accessed September 5, 2013. NLP Identifier: NCT01174550
24. American College of Radiology Imaging Network. Randomized Evaluation of Patients with Stable Angina Comparing Diagnostic Examinations (RESCUE). In: ClinicalTrials.gov Bethesda, MD: National Library of Medicine (US). 2000- Available at: http://clinicaltrials.gov/show/NCT01262625 . Accessed September 5, 2013. NLP Identifier: NCT01262625
25. Quinn G.P., Bell B.A., Bell M.Y., et. al.: The guinea pig syndrome: Improving clinical trial participation among thoracic patients. J Thorac Oncol 2007; 2: pp. 191-196.
26. Likert R.: A technique for the measurement of attitudes. Arch Psychol 1932; 22: pp. e55.
27. Passamani E.: Clinical trials—Are they ethical?. N Engl J Med 1991; 324: pp. 1589-1591.
28. Dunn L.B., Gordon N.E.: Improving informed consent and enhancing recruitment for research by understanding economic behavior. JAMA 2005; 293: pp. 609-612.
29. Siegrist M., Cvetkovich G.: Perception of hazards: The role of social trust and knowledge. Risk Anal 2000; 20: pp. 713-719.
30. Brewin C.R., Bradley C.: Patient preferences and randomized clinical trials. BMJ 1989; 299: pp. 313-315.
31. Bentley J.P., Thacker P.G.: The influence of risk and monetary payment on the research participation decision making process. J Med Ethics 2003; 30: pp. 293-298.