Rationale and Objectives
Many patients with systemic lupus erythematosus (SLE) manifest the recurrence of new brain lesions on follow-up magnetic resonance imaging (MRI) scans. We assessed whether the initial MRI findings help to predict the subsequent development of brain lesions in patients with SLE.
Materials and Methods
We enrolled 64 patients with SLE who had undergone initial and follow-up MRI studies. Two radiologists reviewed and categorized the initial MRI findings and divided the patients into those with no lesions on the initial and follow-up MRI scans (group A, n = 18), those with lesions on the initial scans only (group B, n = 32), and those with lesions on the first and new lesions on the follow-up MRI scans (group C, n = 14). We then looked for independent predictors of the subsequent development of brain lesions, such as antiphospholipid syndrome (APS) and findings on the initial MRI studies.
Results
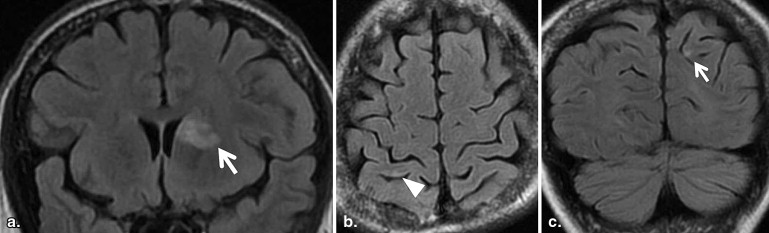
The incidence of lacunar and localized cortical infarcts was significantly greater in group C than group B (50% vs. 0%, P < .001 and 50% vs. 9%, P < .05, respectively). Multivariate logistic regression analysis indicated that lacunar or localized cortical infarcts on the initial MRI scans were independent predictors of the subsequent development of brain lesions (odds ratio [OR]: 5.412, 95% confidence interval [CI]: 1.18–24.85, P = .03), whereas the presence of APS was not (OR: 0.621, 95% CI: 0.18–2.19).
Conclusions
The presence of lacunar and/or localized cortical infarcts on initial MRI scans may predict the development of new brain lesions in patients with SLE.
Systemic lupus erythematosus (SLE) is an autoimmune disease that frequently involves the central nervous system (CNS, . Neuropsychiatric (NP) SLE has been reported in as many as 30%–56% of all patients with SLE , and the quality of life is poorer in patients with NPSLE than without NPSLE . However, the etiology of and basis for NPSLE-associated brain lesions remain uncertain .
The development of NPSLE-associated brain lesions is related to disease activity and severity; one of the most important risk factors for brain lesions is the presence of antiphospholipid syndrome (APS) and/or anticardiolipin antibodies (aPL, . Kaichi et al. showed that abnormal magnetic resonance imaging (MRI) findings were more common in SLE patients with APS than without APS. The recognition of APS is critical for appropriate therapy. Antiplatelet and/or anticoagulation therapy is recommended for NPSLE related to aPL, especially in patients with thrombotic cerebrovascular disease (CVD, . SLE patients without aPL are also potentially at risk for the recurrence of CVD .
Get Radiology Tree app to read full this article<
Materials and methods
Patient Selection
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Image Acquisition
Get Radiology Tree app to read full this article<
Image Interpretations
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Table 1
Demographic and Clinical Characteristics of Patients with SLE
Factor Group A ( N = 18) Group B ( N = 32) Group C ( N = 14)P Value (B/C) Mean age, (year ± SD) 28.8 ± 19.2 32.8 ± 18.8 37.1 ± 16.1 .19 Sex .51 Female [ n (%)] 16 (89) 28 (88) 13 (93) Male [ n (%)] 2 (11) 4 (12) 1 (7) APS positive [ n (%)] 3 (17) 7 (22) 10 (71) <.05 Activity index SLEDAI 13 (10–27) 6 (0–27) 13 (0–28) .28 BILAG Index 20 (15–35) 8.5 (0–21) 7 (4–28) .33 Observation period, (month ± SD) 35.5 ± 35.5 46.9 ± 33.9 26.1 ± 47.9 <.05 Vascular risk factors Hypertension [ n (%)] 11 (61) 1 (3) 3 (21) .08 Hyperlipidemia [ n (%)] 2 (11) 4 (13) 1 (7) .51 Diabetes mellitus [ n (%)] 2 (11) 0 (0) 0 (0) — Smoking [ n (%)] 2 (11) 2 (6) 4 (29) .06 Obesity [ n (%)] 1 (6) 3 (9) 1 (7) .51 Duration of SLE, year: median 5 (1–23) 6 (1–27) 11 (1–23) .24 Treatment Corticosteroids [ n (%)] 18 (100) 30 (94) 14 (100) 0.48 Immunomodulatory drugs [ n (%)] 15 (83) 26 (78) 11 (79) 0.65
APS, antiphospholipid syndrome; BILAG Index, British Isles Lupus Assessment Group Index; SD, standard deviation; SLE, systemic lupus erythematosus; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; Observational period, time interval between initial and final follow-up magnetic resonance imaging.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 2
Comparison of Initial MRI Findings with Groups B and C
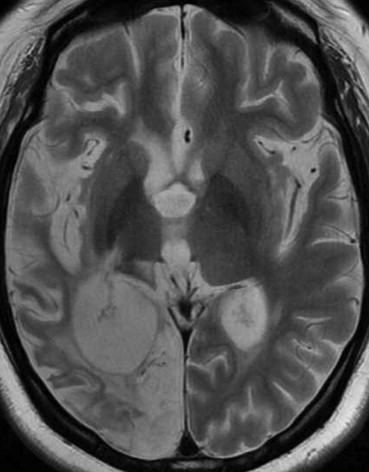
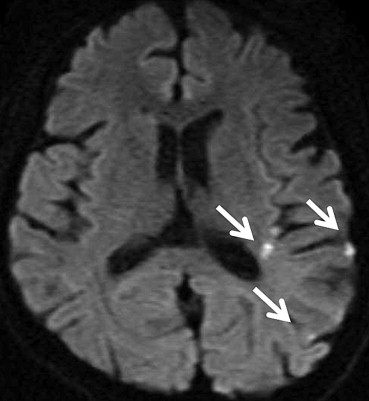
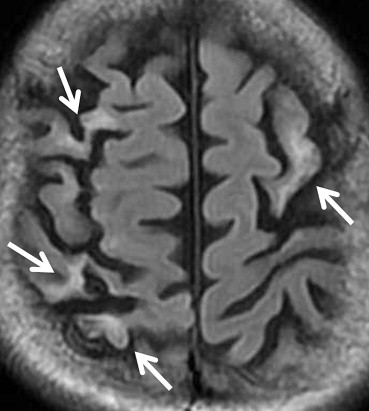
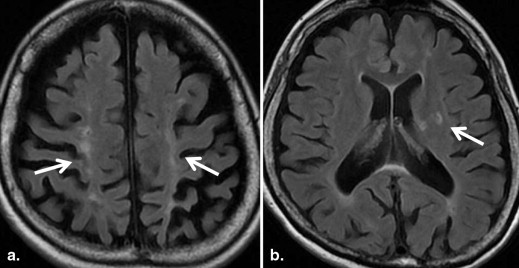
MRI Findings Group B, N = 32; [ n (%)] Group C, N = 14; [ n (%)]P Value White matter hyperintensities 21 (66) 9 (64) .59 Large territorial infarctions 7 (22) 5 (36) .26 Lacunar infarctions 0 (0) 7 (50) <.001 Localized cortical infarctions 3 (9) 7 (50) <.05 Borderzone infarctions 2 (6) 3 (21) .16 Microembolisms 0 (0) 0 (0) — Basal ganglia lesions 2 (6) 1 (7) .67 Hemorrhages 0 (0) 0 (0) — Callosal lesions 0 (0) 0 (0) — Arterial occlusion/stenosis 2 (6) 1 (7) .65
MRI, magnetic resonance imaging.
Table 3
Factors Affecting the Subsequent Development of Brain Lesions: Multivariate Analysis
Factor Odds Ratio 95% Confidence Interval_P_ Value APS positive 0.621 0.18–2.19 .459 Lacunar or localized cortical infarctions 5.412 1.18–24.85 .03
APS, antiphospholipid syndrome.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Johnson R.T., Richardson E.P.: The neurological manifestations of systemic lupus erythematosus. Medicine (Baltimore) 1968; 47: pp. 337-369.
2. O’Connor J.F., Musher D.M.: Central nervous system involvement in systemic lupus erythematosus. A study of 150 cases. Arch Neurol 1966; 14: pp. 157-164.
3. Bertsias G.K., Ioannidis J.P., Aringer M., et. al.: EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis 2010; 69: pp. 2074-2082.
4. Unterman A., Nolte J.E., Boaz M., et. al.: Neuropsychiatric syndromes in systemic lupus erythematosus: a meta-analysis. Semin Arthritis Rheum 2011; 41: pp. 1-11.
5. Hanly J.G., Urowitz M.B., Su L., et. al.: Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Ann Rheum Dis 2010; 69: pp. 529-535.
6. Hanly J.G., Su L., Farewell V., et. al.: Prospective study of neuropsychiatric events in systemic lupus erythematosus. J Rheumatol 2009; 36: pp. 1449-1459.
7. Sibbitt W.L., Brooks W.M., Kornfeld M., et. al.: Magnetic resonance imaging and brain histopathology in neuropsychiatric systemic lupus erythematosus. Semin Arthritis Rheum 2010; 40: pp. 32-52.
8. Kim J.H., Choi C.G., Choi S.J., et. al.: Primary antiphospholipid antibody syndrome: neuroradiologic findings in 11 patients. Korean J Radiol 2000; 1: pp. 5-10.
9. Vadacca M., Buzzulini F., Rigon A., et. al.: [Neuropsychiatric lupus erythematosus]. Reumatismo 2006; 58: pp. 177-186.
10. Govoni M., Bombardieri S., Bortoluzzi A., et. al.: Factors and comorbidities associated with first neuropsychiatric event in systemic lupus erythematosus: does a risk profile exist? A large multicentre retrospective cross-sectional study on 959 Italian patients. Rheumatology (Oxford) 2012; 51: pp. 157-168.
11. Kaichi Y., Kakeda S., Moriya J., et. al.: Brain MR findings in patients with systemic lupus erythematosus with and without antiphospholipid antibody syndrome. AJNR Am J Neuroradiol 2013;
12. Harris E.N., Pierangeli S.S.: Primary, secondary, and catastrophic antiphospholipid syndrome: what’s in a name?. Semin Thromb Hemost 2008; 34: pp. 219-226.
13. Erkan D., Harrison M.J., Levy R., et. al.: Aspirin for primary thrombosis prevention in the antiphospholipid syndrome: a randomized, double-blind, placebo-controlled trial in asymptomatic antiphospholipid antibody-positive individuals. Arthritis Rheum 2007; 56: pp. 2382-2391.
14. Bernatsky S., Clarke A., Gladman D.D., et. al.: Mortality related to cerebrovascular disease in systemic lupus erythematosus. Lupus 2006; 15: pp. 835-839.
15. Hochberg M.C.: Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: pp. 1725.
16. Bombardier C., Gladman D.D., Urowitz M.B., et. al.: Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 1992; 35: pp. 630-640.
17. Symmons D.P., Coppock J.S., Bacon P.A., et. al.: Development and assessment of a computerized index of clinical disease activity in systemic lupus erythematosus. Members of the British Isles Lupus Assessment Group (BILAG). Q J Med 1988; 69: pp. 927-937.
18. Stoll T., Stucki G., Malik J., et. al.: Further validation of the BILAG disease activity index in patients with systemic lupus erythematosus. Ann Rheum Dis 1996; 55: pp. 756-760.
19. Lodder J., Hupperts R., Boreas A., et. al.: The size of territorial brain infarction on CT relates to the degree of internal carotid artery obstruction. J Neurol 1996; 243: pp. 345-349.
20. Bang O.Y., Ovbiagele B., Liebeskind D.S., et. al.: Clinical determinants of infarct pattern subtypes in large vessel atherosclerotic stroke. J Neurol 2009; 256: pp. 591-599.
21. Kimura K., Minematsu K., Koga M., et. al.: Microembolic signals and diffusion-weighted MR imaging abnormalities in acute ischemic stroke. AJNR Am J Neuroradiol 2001; 22: pp. 1037-1042.
22. Bogousslavsky J., Regli F.: Unilateral watershed cerebral infarcts. Neurology 1986; 36: pp. 373-377.
23. Damasio H.: A computed tomographic guide to the identification of cerebral vascular territories. Arch Neurol 1983; 40: pp. 138-142.
24. Fisher C.M.: Lacunar strokes and infarcts: a review. Neurology 1982; 32: pp. 871-876.
25. Fazekas F., Kleinert R., Offenbacher H., et. al.: The morphologic correlate of incidental punctate white matter hyperintensities on MR images. AJNR Am J Neuroradiol 1991; 12: pp. 915-921.
26. Wuerfel J., Haertle M., Waiczies H., et. al.: Perivascular spaces–MRI marker of inflammatory activity in the brain?. Brain 2008; 131: pp. 2332-2340.
27. Chao C.P., Kotsenas A.L., Broderick D.F.: Cerebral amyloid angiopathy: CT and MR imaging findings. Radiographics 2006; 26: pp. 1517-1531.
28. Sabet A., Sibbitt W.L., Stidley C.A., et. al.: Neurometabolite markers of cerebral injury in the antiphospholipid antibody syndrome of systemic lupus erythematosus. Stroke 1998; 29: pp. 2254-2260.
29. Atsumi T., Khamashta M.A., Haworth R.S., et. al.: Arterial disease and thrombosis in the antiphospholipid syndrome: a pathogenic role for endothelin 1. Arthritis Rheum 1998; 41: pp. 800-807.
30. Duvernoy H.M., Delon S., Vannson J.L.: Cortical blood vessels of the human brain. Brain Res Bull 1981; 7: pp. 519-579.
31. Hughes G.R., Khamashta M.A.: Seronegative antiphospholipid syndrome. Ann Rheum Dis 2003; 62: pp. 1127.
32. Rodriguez-Garcia J.L., Bertolaccini M.L., Cuadrado M.J., et. al.: Clinical manifestations of antiphospholipid syndrome (APS) with and without antiphospholipid antibodies (the so-called ‘seronegative APS’). Ann Rheum Dis 2012; 71: pp. 242-244.
33. Erkan D., Yazici Y., Peterson M.G., et. al.: A cross-sectional study of clinical thrombotic risk factors and preventive treatments in antiphospholipid syndrome. Rheumatology (Oxford) 2002; 41: pp. 924-929.