Rationale and Objectives
The aim of this study was to assess the feasibility of thermally cross-linked superparamagnetic iron oxide nanoparticle contrast (TCL-SPION) in magnetic resonance (MR) imaging (MRI) for the detection of lymph node metastasis in experimental model.
Materials and Methods
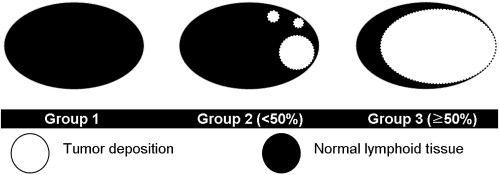
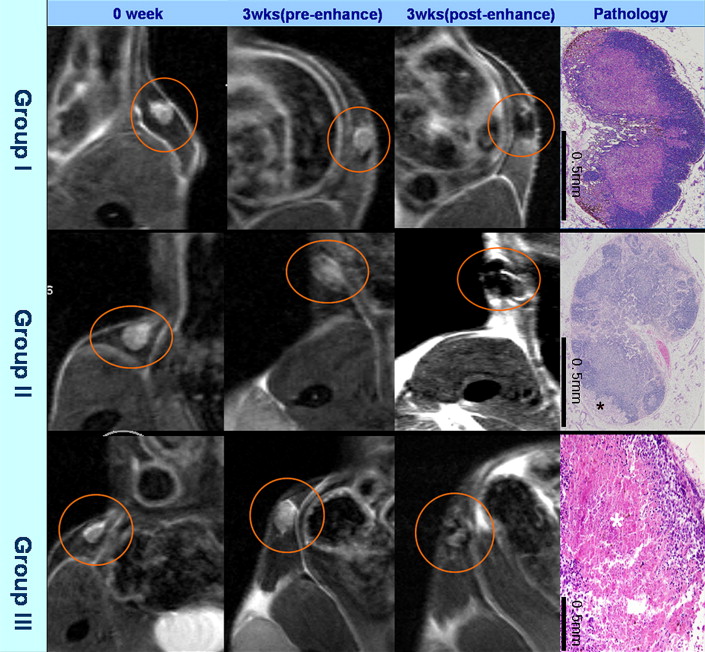
B16F1 human melanoma cells were subcutaneously injected into the thighs of C57BL/6 mice (n = 10). MRI was performed 21 days after tumor injection using a 4.7-T MR scanner. In vivo MRI was performed before and after the intravenous administration of TCL-SPION using T2 fast spin-echo and T2 gradient-echo pulse sequences. Then, ex vivo MR images were obtained for resected inguinal lymph nodes (n = 18) using the same pulse sequences as for in vivo imaging. On the basis of hematoxylin and eosin staining results, the lymph nodes were classified into three groups: group 1, nonmetastatic; group 2, tumor volume <50% of the resected sample; and group 3, tumor volume >50% of the resected sample. Size, signal-to-background ratio, and enhancement pattern were evaluated in each of the three groups on ex vivo images.
Results
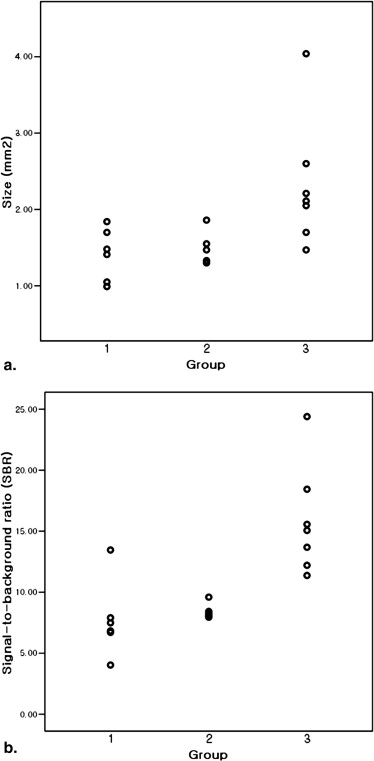
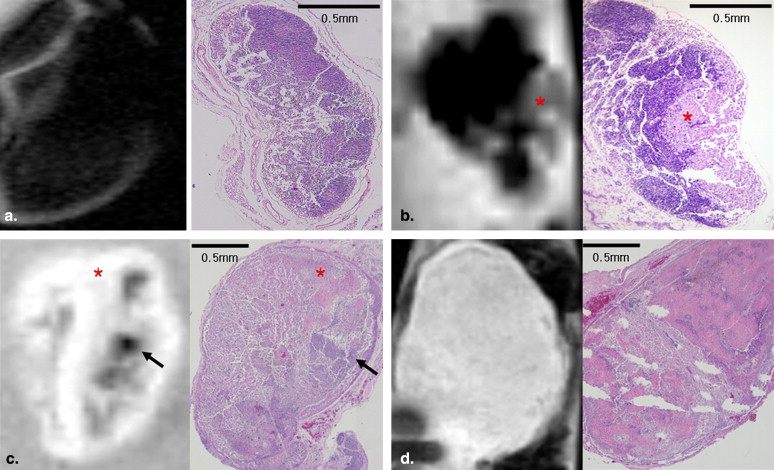
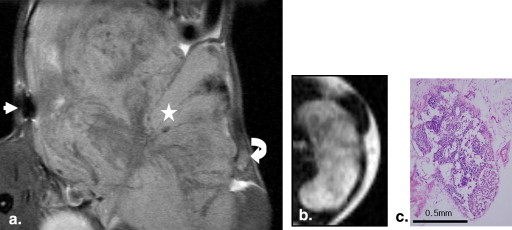
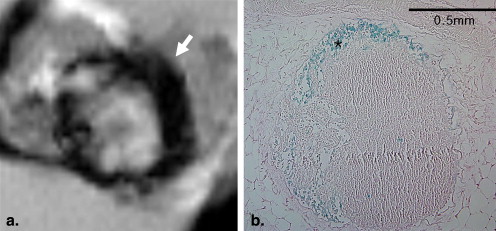
The findings observed on ex vivo MR images of 18 inguinal lymph nodes were compared with histopathologic findings. All nodes were classified into three groups: group 1, n = 6; group 2, n = 5; and group 3, n = 7. The sizes of the lymph nodes in group 1 were significantly different from the sizes of those in group 3 ( P = .014), but there was no significant difference in lymph node sizes between groups 1 and 2 ( P = .792). Signal-to-background ratios of samples in groups 2 and 3 were significantly higher than those of samples in group 1 ( P = .045 and P = .007, respectively). Each group of lymph nodes showed characteristic enhancement patterns that were well correlated between the images and pathology, except for one node.
Conclusions
The features and extent of metastasis in the lymph nodes corresponded to those observed on TCL-SPION-enhanced MR images. TCL-SPION-enhanced MRI is useful for the detection and estimation of lymph node metastasis.
The detection of metastasis to local or distant lymph nodes is very important to predict patient outcomes and plan treatment. The survival rates of patients with malignant metastatic lymph nodes differ significantly, despite small extent of metastasis . Despite efforts to establish various criteria, thus far, only nodal size is used as a criterion for the assessment of nodal metastasis using conventional cross-sectional imaging modalities such as computed tomography and magnetic resonance (MR) imaging (MRI). However, size criteria showed low sensitivity (50%–70%) in the detection of nodal metastasis, particularly in the detection of small metastasis .
MRI enhanced with superparamagnetic iron oxide (SPIO) and ultrasmall SPIO (USPIO) nanoparticles has been known to be useful for the detection of metastatic tumors in lymph nodes, particularly for the detection of occult small metastases. Superparamagnetic nanoparticles are phagocytosed by reticuloendothelial cells in normal lymphoid tissues, but not in tumor tissue, because they lack reticuloendothelial cells. The nanoparticles induce a signal drop in lymphoid tissues on T2-weighted images because of their superparamagnetic effect. Therefore, metastatic tumors can be differentiated from normal lymphoid tissues using superparamagnetic nanoparticle–enhanced MRI. Various studies have shown the usefulness of SPIO and USPIO nanoparticles in the detection of metastatic lymph nodes on MRI. Recently, various types of USPIO nanoparticles have been developed for the purpose of lymph node imaging, particularly for the detection of metastatic lymph nodes . However, not all USPIO nanoparticles are suitable for nodal imaging. A long half-life of nanoparticles in blood is essential for lymph node imaging, and the size of nanoparticles is a major factor that determines their half-life in blood. Smaller nanoparticles usually have longer blood half-lives than larger nanoparticles .
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
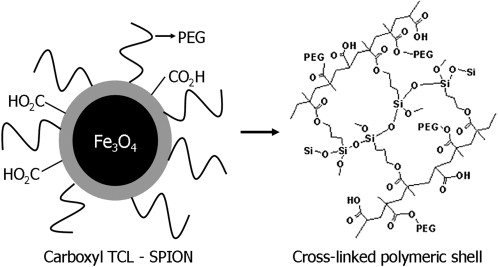
TCL-SPION
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Animal Model of Lymph Node Metastases
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
MRI
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Histopathology
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Image Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Histopathology
Get Radiology Tree app to read full this article<
MRI Analysis
Nodal Size
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
SBR
Get Radiology Tree app to read full this article<
Nodal Enhancement Pattern After TCL-SPION Administration
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Dowlatshahi K., Fan M., Snider H.C., et. al.: Lymph node micrometastases from breast carcinoma: reviewing the dilemma. Cancer 1997; 80: pp. 1188-1197.
2. Herrera-Ornelas L., Justiniano J., Castillo N., et. al.: Metastases in small lymph nodes from colon cancer. Arch Surg 1987; 122: pp. 1253-1256.
3. Harisinghani M.G., Barentsz J., Hahn P.F., et. al.: Noninvasive detection of clinically occult lymph node metastases in prostate cancer. N Engl J Med 2003; 348: pp. 2491-2499.
4. Mack M.G., Balzer J.O., Straub R., et. al.: Superparamagnetic iron oxide-enhanced MR imaging of head and neck lymph nodes. Radiology 2002; 222: pp. 239-244.
5. Harisinghani M.G., Saini S., Hahn P.F., et. al.: MR imaging of lymph nodes in patients with primary abdominal and pelvic malignancies using ultrasmall superparamagnetic iron oxide (Combidex). Acad Radiol 1998; 5: pp. S167-S169.
6. Raynal I., Prigent P., Peyramaure S., et. al.: Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10. Invest Radiol 2004; 39: pp. 56-63.
7. McLachlan S.J., Morris M.R., Lucas M.A., et. al.: Phase I clinical evaluation of a new iron oxide MR contrast agent. J Magn Reson Imaging 1994; 43: pp. 301-307.
8. Wunderbaldinger P., Josephson L., Weissleder R.: Crosslinked iron oxides (CLIO): a new platform for the development of targeted MR contrast agents. Acad Radiol 2002; 9: pp. S304-S306.
9. Lee H., Yu M.K., Park S., et. al.: Thermally cross-linked superparamagnetic iron oxide nanoparticles: synthesis and application as a dual imaging probe for cancer in vivo. J Am Chem Soc 2007; 129: pp. 12739-12745.
10. Lee H., Lee E., Kim D.K., et. al.: Antibiofouling polymer-coated superparamagnetic iron oxide nanoparticles as potential magnetic resonance contrast agents for in vivo cancer imaging. J Am Chem Soc 2006; 128: pp. 7383-7389.
11. Gupta A.K., Gupta M.: Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005; 26: pp. 3995-4021.
12. Mikhaylova M., Kim D.K., Bobraysheva N., et. al.: Superparamagnetism of magnetite nanoparticles: dependence on surface modification. Langmuir 2004; 20: pp. 2472-2477.
13. Hart I.R., Fidler I.J.: Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Res 1980; 40: pp. 2281-2287.
14. Fidler I.J.: Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res 1975; 35: pp. 218-224.
15. Koh D.M., Brown G., Temple L., et. al.: Rectal cancer: mesorectal lymph nodes at MR imaging with USPIO versus histopathologic findings. Radiology 2004; 231: pp. 91-99.
16. Wunderbaldinger P., Josephson L., Bremer C.: Detection of lymph node metastases by contrast-enhanced MRI in an experimental model. Mag Reson Med 2002; 47: pp. 292-297.
17. Memarsadeghi M., Riedl C.C., Kaneider A., et. al.: Axillary lymph node metastases in patients with breast carcinomas: assessment with nonenhanced versus USPIO-enhanced MR imaging. Radiology 2006; 241: pp. 367-377.
18. Bellin M.F., Roy C., Kinkel K., et. al.: Lymph node metastases: safety and effectiveness of MR imaging with ultrasmall superparamagnetic iron oxide particles-initial clinical experience. Radiology 1998; 207: pp. 799-808.
19. Corot C., Robert P., Idée L.M., et. al.: Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev 2006; 58: pp. 1471-1504.
20. Kohler N., Fryxell G.E., Zhang M.: A bifunctional poly (ethylene glycol) silane immobilized on metallic oxide-based nanoparticles for conjugation with cell targeting agents. J Am Chem Soc 2004; 126: pp. 7206-7211.
21. Yu M.K., Jeong Y.Y., Park J., et. al.: Drug-loaded superparamagnetic iron oxide nanoparticles for combined cancer imaging and therapy in vivo. Angew Chem Int Ed 2008; 47: pp. 5362-5365.
22. Kim D.K., Mikhaylova M., Wang F.H., et. al.: Starch-coated superparamagnetic nanoparticles as MR contrast agents. Chem Mat 2003; 15: pp. 4343-4351.
23. Wang Y.X., Hussain S.M., Krestin G.P.: Superparamagnetic iron oxide contrast agents: physiochemical characteristics and applications in MR imaging. Eur Radiol 2001; 11: pp. 2319-2331.
24. Cho W.S., Cho M., Kim S.R., et. al.: Pulmonary toxicity and kinetic study of Cy5.5-conjugated superparamagnetic iron oxide nanoparticles by optical imaging. Toxicol Appl Pharmacol 2009; 239: pp. 106-115.
25. Weissleder R., Elizondo G., Wittenberg J., et. al.: Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology 1990; 175: pp. 494-498.
26. Garlick D.G., Renkin E.M.: Transport of large molecules from plasma to intestitial fluid and lymph in dogs. Am J Physiol 1970; 219: pp. 1595-1605.
27. Ogisho Y., Matsuoka O.: Time dependent changes of microscopic localization of intravenously administered colloidal carbon particles in mouse lymph nodes. J Toxicol Sci 1983; 8: pp. 291-300.
28. Lind K., Kresse M., Debus N.P., et. al.: A novel formulation for superparamagnetic iron oxide (SPIO) particles enhancing MR lymphography: comparison of hysicochemical properties and the in vivo behaviour. J Drug Target 2002; 10: pp. 221-230.
29. Wagner S., Pfefferer D., Ebert W., et. al.: Intravenous MR lymphography with superparamagnetic iron oxide particles: experimental studies in rats and rabbits. Eur Radiol 1995; 5: pp. 640-646.
30. Bourrinet P., Bengele H.H., Bonnemain B., et. al.: Preclinical safety and pharmacokinetic profile of ferumoxtran-10 an ultrasmall superparamagnetic iron oxide magnetic resonance contrast agent. Invest Radiol 2006; 413: pp. 313-324.
31. Wagner S., Schnorr J., Pilgrimm H., et. al.: Monomer-coated very small superparamagnetic iron oxide particles as contrast medium for magnetic resonance imaging: preclinical in vivo characterization. Invest Radiol 2002; 37: pp. 167-177.
32. Weinreb J.C., Brateman L., Babcock E.E., et. al.: Chemical shift artifact in clinical magnetic resonance images at 0.35 T. AJR Am J Roentgenol 1985; 145: pp. 183-185.
33. Frayne R., Goodyear B.G., Dickhoff P., et. al.: Magnetic resonance imaging at 3.0 tesla: challenges and advantages in clinical neurological imaging. Invest Radiol 2003; 38: pp. 385-402.