Rationale and Objectives
The goal was to determine whether the tumor vascular disrupting actions of low-intensity ultrasound were frequency dependent.
Materials and Methods
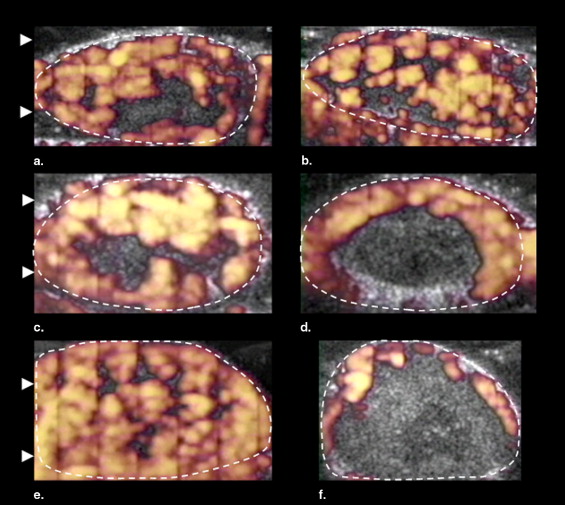
The effect of the frequency (1 MHz at 2.2 W/cm 2 or 3 MHz at 2.4 W/cm 2 ) of low-intensity ultrasound as a neovascular disrupting modality was investigated in 15 murine melanomas (K1735 22 ) insonated for 3 minutes after the intravenous injection of a microbubble contrast agent (Definity). In contrast-enhanced power Doppler observations of each tumor (before and after treatment), measurements were made of the size of the area of the tumor that was perfused with blood containing the ultrasound contrast agent (percentage area of flow [PAF]), and the volume of contrast agent flowing through the unit volume of the tumor (color-weighted fractional area [CWFA]). During insonation of the tumor, the temperature was measured with a fine wire thermocouple in an additional eight mice.
Results
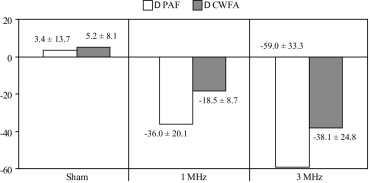
The antivascular action of low-intensity ultrasound was significantly enhanced (PAF by 64%; CWFA by 106%) when the tumor was treated with 3-MHz ultrasound rather than 1 MHz (analysis of variance: PAF, P = .02; CWFA, P = .04). The average rate of tumor temperature increase was 2.6 ± 1.3°C/min for 1 MHz and 5.0 ± 1.7°C/min for 3 MHz; these increases were significantly different ( P = .04).
Conclusions
Insonation of the tumor at a higher frequency amplified the heating of the neoplasm and led to greater disruption of the tumor vasculature; 3-MHz ultrasound was more efficacious than 1 MHz for antivascular cancer therapy.
The influence of low-intensity physiotherapy ultrasound beams on the growth of neoplasms has been investigated. In a rhabdomyosarcoma in mice, it was found that the neoplasms receiving ten 5-minute treatments over 2 weeks with a continuous 3-MHz beam at 1 W/cm 2 grew at a faster rate than the control, untreated tumors ( ). Such an increase in growth may have been related to an enhanced angiogenic response similar to that observed after the insonation (15 or 30 minutes, 1.0 MHz, 20% duty cycle, 2.2 W/cm 2 ) of endothelial cells in tissue culture ( ). Subsequent rhabdomyosarcoma studies showed that 3-MHz beams of 0.75 W/cm 2 (continuous) and 1.5 W/cm 2 (pulsed) had no effect on tumor growth, and at necropsy, there were no histologic changes related to the therapy ( ).
As opposed to sonication alone, the combination of a microbubble-containing contrast agent and low-intensity ultrasound acted as a potent tumor vascular disrupting agent ( ). Treatment of a murine melanoma with a low-intensity beam (1 MHz, continuous, spatial average temporal average intensity (I SATA ) = 2.3 W/cm 2 ) reduced tumor blood flow by 25% for each minute of insonation ( ). Contrast-enhanced Doppler imaging used in this study assesses blood flow by measuring the fractional area of tumor enhanced by the contrast agent and the magnitude of enhancement. These properties of Doppler images are intimately related to fractional blood volume or tumor perfusion; in this work, we have used these terms interchangeably.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Tumor Model and Ultrasonography
Get Radiology Tree app to read full this article<
Tumor Insonation and Experimental Design
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Analysis of Contrast-enhanced Power Doppler Images
Get Radiology Tree app to read full this article<
CWFA=∑i=100i=1iniN. C
W
F
A
=
∑
i
=
1
i
=
100
i
n
i
N
.
Get Radiology Tree app to read full this article<
Because the color level of pixels in the power Doppler image is related to the concentration of moving scatterers, CWFA is a measure of the volume of contrast agent flowing through the unit volume (voxels) of the tumor within the image plane.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
In Vivo Estimations of Tumor Heating
Get Radiology Tree app to read full this article<
ΔT¯¯¯=∫0tΔTidt∫0tdt, Δ
T
¯
=
∫
0
t
Δ
T
i
d
t
∫
0
t
d
t
,
where ΔTi Δ
T
i is the increase in temperature from heating. The peak increase in temperature during each insonation was also recorded.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
TD=∫0tR(T−43)dt, T
D
=
∫
0
t
R
(
T
−
43
)
d
t
,
where TD = thermal dose, R = 2 for T ≥ 43°C, and R = 4 for 37°C < T < 43°C. The mean ± standard deviation of each of the temperature and TD measurements for each frequency were determined and the statistical significance of the difference between frequencies was established by an unpaired t -test assuming unequal variances (MedCalc Software). Each mouse was euthanized at the completion of the experiment.
Get Radiology Tree app to read full this article<
Histology
Get Radiology Tree app to read full this article<
Results
Clinical and Doppler Findings
Get Radiology Tree app to read full this article<
Table 1
Effect of Sonication on Murine Tumor Vascularity (measurements within groups from contrast-enhanced power Doppler Images)
PAF (%) CWFA (%) Group Sonication Frequency Pretreatment Post-treatment_P_ Value Pretreatment Post-treatment_P_ Value 1 Sham 61.9 ± 7.2 65.3 ± 14.9 .61 26.1 ± 7.8 31.3 ± 10.4 .23 2 1 MHz 64.7 ± 21.1 28.8 ± 26.5 .016 32.6 ± 14.4 14.1 ± 15.4 .009 3 3 MHz 69.7 ± 24.0 14.6 ± 12.2 .017 39.7 ± 22.0 6.4 ± 6.3 .027
CWFA: color weighted fractional area; PAF: percentage area of flow.
Data analyzed by paired t -test.
Get Radiology Tree app to read full this article<
In Vivo Estimations of Tumor Heating
Get Radiology Tree app to read full this article<
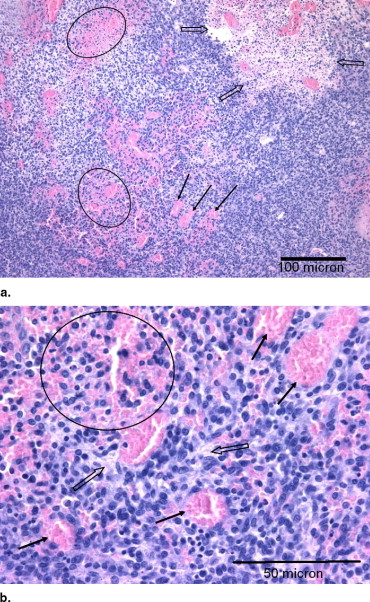
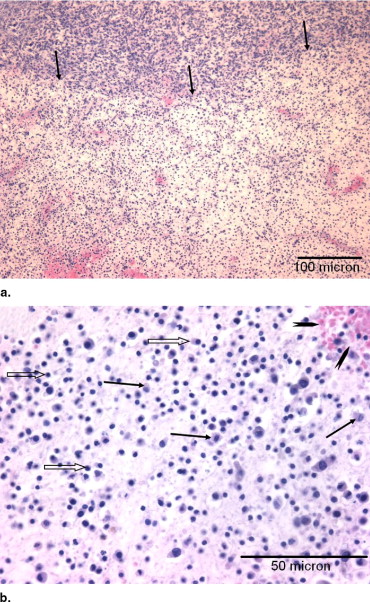
Histologic Findings
Get Radiology Tree app to read full this article<
Table 2
Summary of Histologic Changes in Murine Melanomas
% Area Intercellular Fluid ⁎ Group Dilation Hemorrhage Dissociation Necrosis Cyst % Area Score 1 0.2 ± 0.3 1.5 ± 1.2 1.6 ± 1.7 1.0 ± 1.1 0.3 ± 0.5 12.2 ± 8.5 1.1 ± 0.6 2 33.2 ± 23.0 27.2 ± 20.1 25.4 ± 22.9 6.8 ± 4.5 0.1 ± 0.2 19.6 ± 10.7 1.7 ± 1.0 3 69.0 ± 11.5 45.3 ± 11.3 41.3 ± 22.2 10.2 ± 3.0 1.2 ± 2.7 5.4 ± 4.1 1.0 ± 0.8 Analysis of variance P value 0.0001 0.002 0.03 0.006 0.54 1, 2 P value 0.009 0.02 0.09 0.02 0.89 1, 3 P value <0.0001 0.0007 0.01 0.002 0.41 2, 3 P value 0.004 0.06 0.22 0.14 0.31
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Sicard-Rosenbaum L., Lord D., Danoff J.V., et. al.: Effects of continuous therapeutic ultrasound on growth and metastasis of subcutaneous murine tumors. Phys Ther 1995; 75: pp. 3-11.
2. Mizrahi N., Seliktar D., Kimmel E.: Ultrasound-induced angiogenic response in endothelial cells. Ultrasound Med Biol 2007; 33: pp. 1818-1829.
3. Sicard-Rosenbaum L., Danoff J.V., Guthrie J.A., et. al.: Effects of energy-matched pulsed and continuous ultrasound on tumor growth in mice. Phys Ther 1998; 78: pp. 271-277.
4. Wood A.K.W., Ansaloni S., Ziemer L.S., et. al.: The antivascular action of physiotherapy ultrasound on murine tumors. Ultrasound Med Biol 2005; 31: pp. 1403-1410.
5. Bunte R.M., Ansaloni S., Sehgal C.M., et. al.: Histopathological observations of the antivascular effects of physiotherapy ultrasound on a murine neoplasm. Ultrasound Med Biol 2006; 32: pp. 453-461.
6. Wood A.K.W., Bunte R.M., Cohen J., et. al.: The antivascular action of physiotherapy ultrasound on a murine tumor: role of a microbubble contrast agent. Ultrasound Med Biol 2007; 33: pp. 1901-1910.
7. Carmeliet P., Jain R.K.: Angiogenesis in cancer and other diseases. Nature 2000; 407: pp. 249-257.
8. Fenton R.G., Longo D.L.: Cancer cell biology and angiogenesis.Kasper D.L.Fauci A.S.Longo D.L. et. al.Harrison’s principles of internal medicine.2005.McGraw-HillNew York:pp. 453-464.
9. Haroon Z.A., Peters K.G., Greenberg C.S., et. al.: Angiogenesis and oxygen transport in solid tumors.Teicher B.A.Antiangiogenic agents in cancer therapy.1999.Humana PressTotowa, NJ:pp. 3-21.
10. Jain R.K.: Tumor angiogenesis and accessibility: role of vascular endothelial growth factor. Semin Oncol 2002; 29: pp. 3-9.
11. Holt R.G., Roy R.A.: Measurements of bubble-enhanced heating from focused, MHz-frequency ultrasound in tissue-mimicking material. Ultrasound Med Biol 2001; 27: pp. 1399-1412.
12. Robinson T.C., Lele P.P.: An analysis of lesion development in the brain and in plastics by high-intensity focused ultrasound at low-megahertz frequencies. J Acoust Soc Am 1972; 51: pp. 1333-1351.
13. Bamber J.C., Hill C.R.: Ultrasonic attenuation and propagation speed in mammalian tissues as a function of temperature. Ultrasound Med Biol 1979; 5: pp. 149-157.
14. Bamber J.C.: Attenuation and absorption (Section 4.5.2.5, Temperature dependence).Hill C.R.Physical principles of medical ultrasonics.1986.Ellis Horwood LimitedChichester, UK:pp. 183-185.
15. Razansky D., Einziger P.D., Adam D.R.: Enhanced heat deposition using ultrasound contrast agent—modelling and experimental observations. IEEE Trans Ultrason Ferroelectr Freq Control 2006; 53: pp. 137-147.
16. Umemura S., Kawabata K., Sasaki K.: In vivo acceleration of ultrasonic tissue heating by microbubble agent. IEEE Trans Ultrason Ferroelectr Freq Control 2005; 52: pp. 1690-1698.
17. Hill C.R.: Ultrasonic exposure thresholds for changes in cells and tissues. J Acoust Soc Am 1972; 52: pp. 667-672.
18. ter Haar G.R.: Ultrasonic biophysics (Section 12.2, Cavitation).Hill C.R.Physical principles of medical ultrasonics.1986.Ellis Horwood LimitedChichester, UK:pp. 388-408.
19. Kamotani Y., Lee W.M., Arger P.H., et. al.: Multigated contrast-enhanced power Doppler to measure blood flow in mice tumors. Ultrasound Med Biol 2003; 29: pp. 977-984.
20. Sehgal C.M., Arger P.H., Rowling S.E., et. al.: Quantitative vascularity of breast masses by Doppler imaging: regional variations and diagnostic implications. J Ultrasound Med 2000; 19: pp. 427-440.
21. Sehgal C.M., Arger P.H., Silver A.C., et. al.: Renal blood flow changes induced with endothelin-1 and fenoldopam mesylate at quantitative Doppler US: initial results in a canine study. Radiology 2001; 219: pp. 419-426.
22. Liu H.L., Chang H., Chen W.S., et. al.: Feasibility of transrib focused ultrasound thermal ablation for liver tumors using a spherically curved 2D array: a numerical study. Med Phys 2007; 34: pp. 3436-3448.
23. Dewey W.C.: Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 1994; 10: pp. 457-483.
24. Dudar T.E., Jain R.K.: Differential response of normal and tumor microcirculation to hyperthermia. Cancer Res 1984; 44: pp. 605-612.
25. Song C.W.: Effect of hyperthermia on vascular functions of normal tissues and experimental tumors; brief communication. J Natl Cancer Inst 1978; 60: pp. 711-713.
26. Emami B., Nussbaum G.H., TenHaken R.K., et. al.: Physiological effects of hyperthermia: response of capillary blood flow and structure to local tumor heating. Radiology 1980; 137: pp. 805-809.
27. Eddy H.A., Chmielewski G.: Effect of hyperthermia, radiation and Adriamycin combinations on tumor vascular function. Int J Radiat Oncol Biol Phys 1982; 8: pp. 1167-1175.
28. Onishi T., Machida T., Iizuka N., et. al.: Influence of differences in tumor vascularity upon the effects of hyperthermia. Urol Res 1990; 182: pp. 313-318.
29. Chen Q., Tong S., Dewhirst M.W., et. al.: Targeting tumor microvessels using doxorubicin encapsulated in a novel thermosensitive liposome. Mol Cancer Ther 2004; 3: pp. 1311-1317.
30. Baker K.G., Robertson V.J., Duck F.A.: A review of therapeutic ultrasound: biophysical effects. Phys Ther 2001; 81: pp. 1351-1358.
31. Goss S.A., Johnston R.L., Dunn F.: Comprehensive compilation of empirical ultrasonic properties of mammalian tissues. J Acoust Soc Am 1978; 64: pp. 423-457.
32. Goss S.A., Frizzell L.A., Dunn F.: Frequency dependence of ultrasonic absorption in mammalian testis. J Acoust Soc Am 1978; 63: pp. 1226-1229.
33. Draper D.O., Castel J.C., Castel D.: Rate of temperature increase in human muscle during 1MHz and 3MHz continuous ultrasound. J Orthop Sports Phys Ther 1995; 22: pp. 142-150.
34. Fry F.J., Kossoff G., Eggleton R.C., et. al.: Threshold ultrasonic dosages for structural changes in the mammalian brain. J Acoust Soc Am 1970; 48: pp. 1413-1417.
35. Dunn F., Lohnes J.E., Fry F.J.: Frequency dependence of threshold ultrasonic dosages for irreversible structural changes in mammalian brain. J Acoust Soc Am 1975; 58: pp. 512-514.
36. Hynynen K.: The threshold for thermally significant cavitation in dog’s thigh muscle in vivo. Ultrasound Med Biol 1991; 17: pp. 157-169.
37. Clarke R.L., ter Haar G.R.: Temperature rise recorded during lesion formation by high-intensity focused ultrasound. Ultrasound Med Biol 1997; 23: pp. 299-306.
38. Miller D.L., Thomas R.M.: Ultrasound contrast agents nucleate inertial cavitation in vitro. Ultrasound Med Biol 1995; 21: pp. 1059-1065.
39. Tu J., Hwang J.H., Matula T.J., et. al.: Intravascular inertial cavitation activity detection and quantification in vivo with Optison. Ultrasound Med Biol 2006; 32: pp. 1601-1609.