Rationale and Objectives
The development of parallel magnetic resonance imaging has resulted in the frequent use of diffusion-weighted imaging (DWI) in clinical medicine, which usually involves the use of contrast medium. However, gadolinium (Gd) contrast medium may have some effect on DWI and the apparent diffusion coefficient (ADC). The present study was performed to determine whether the magnetic susceptibility of contrast medium alters the DWI signal and the value of ADC in some imaging techniques.
Materials and Methods
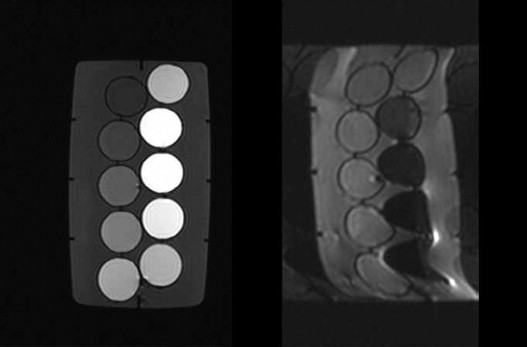
Nonfat suppression DWI, short-time inversion recovery (STIR) combination, and chemical shift selective (CHESS) combination DWI were performed to examine 10 phantoms with gadolinium-meglumine gadopentetate (Gd-DTPA) dissolved at concentrations from 0.0005 to 0.1 mmol in physiologic saline as a contrast medium. The average pixel value and ADC of each method were determined.
Results
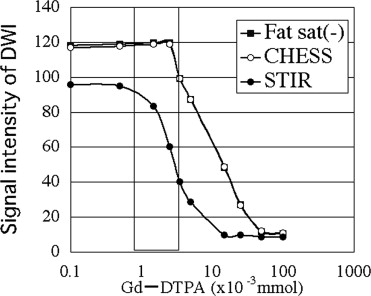
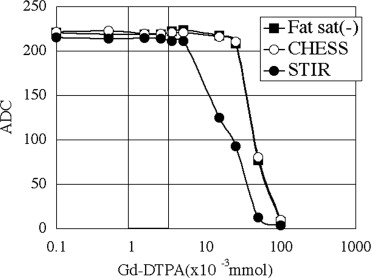
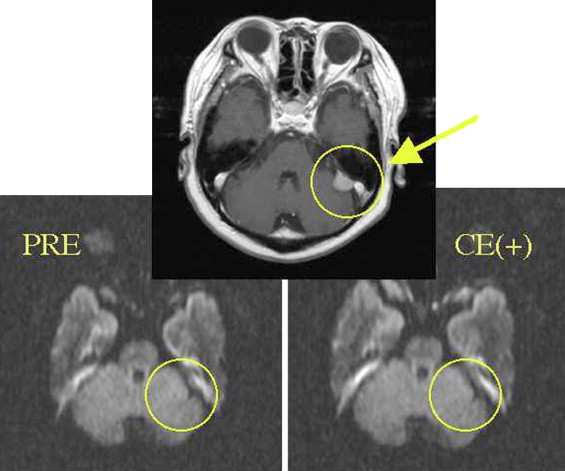
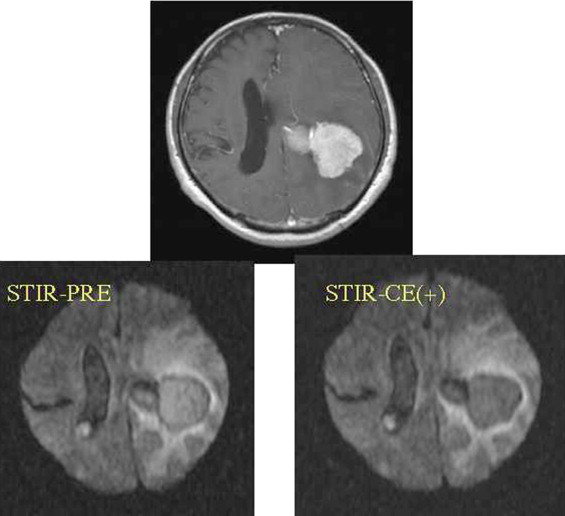
ADC showed no differences between before and after treatment with contrast medium for all imaging techniques with Gd considered distributed over the whole tumor. The signal intensity did not change on nonfat suppression or CHESS combination DWI, but deteriorated on STIR.
Conclusions
ADC was not influenced by the magnetic susceptibility of contrast medium. In addition, it was suggested that the ability of tumor detection may be reduced if STIR is used as fat suppression.
Diffusion-weighted imaging (DWI) has contributed to diagnosis as a new complement to magnetic resonance imaging. In particular, it has become the most important imaging technique in the diagnosis of acute stroke. With the recent development of parallel magnetic resonance imaging, DWI has often been used in clinical medicine to both search for tumors and as a discrimination diagnosis ( ).
However, the application of DWI in routine clinical examinations has not been established; therefore, DWI is often performed after use of contrast medium. There is concern regarding whether gadolinium (Gd) contrast medium influences the DWI signal and the value of the apparent diffusion coefficient (ADC).
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Takahara T., Imai Y., Yamashita T., et. al.: Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med 2004; 22: pp. 275-282.
2. Nasu K., Kuroki Y., Nawano S., et. al.: Hepatic metastases: diffusion weighted sensitivity-encoding versus SPIO-enhanced MR imaging. Radiology 2006; 239: pp. 122-130.
3. Moteki T., Ishizuka H.: Diffusion-weighted EPI of cystic ovarian lesions: evaluation of cystic contents using apparent diffusion coefficients. J Magn Reson Imaging 2000; 12: pp. 1014-1019.
4. Moteki T., Horikoshi H., Endo K.: Diffusion coefficient and signal intensity in endometrial and other pelvic cysts. Magn Reson Imaging 2002; 20: pp. 463-470.
5. Sato C., Naganawa S., Nakamura T., et. al.: Differentiation of noncancerous tissue and cancer lesions by apparent diffusion coefficient values in transition and peripheral zones of the prostate. J Magn Reson Imaging 2005; 21: pp. 258-262.
6. Shimofusa R., Fujimoto H., Akamoto H., et. al.: Diffusion-weighted imaging of prostate cancer. J Compt Assist Tomogr 2005; 29: pp. 149-153.
7. Naganawa S., Sato C., Kumada H., et. al.: Apparent diffusion coefficient in cervical cancer of the uterus: comparison with the normal uterine cervix. Eur Radiol 2005; 15: pp. 71-78.
8. Hosseinzadeh K., Schwarz S.D.: Endorectal diffusion-weighted imaging in prostate cancer to differentiate malignant and benign peripheral zone tissue. J Magn Reson Imaging 2004; 20: pp. 654-661.
9. Naganawa S., Kawai H., Fukatsu H., et. al.: Diffusion-weighted imaging of the liver: technical challenges and prospects for the future. Magn Reson Med Sci 2005; 4: pp. 175-186.
10. Pickles MD, Gibbs P, Sreenivas M, et al. Diffusion-weighted imaging of normal and malignant prostate tissue at 3T. J Magn Reson Imaging 20006; 23:130–134.
11. Koinuma M., Ohashi I., Hanafusa K., et. al.: Apparent diffusion coefficient measurements with diffusion-weighted magnetic resonance imaging for evaluation of hepatic fibrosis. J Magn Reson Imaging 2005; 22: pp. 80-85.
12. Sun X.J., Quan X.Y., Huang F.H., et. al.: Quantitative evaluation of diffusion-weighted magnetic resonance imaging of focal hepatic lesions. World J Gastroenterol 2005; 11: pp. 6535-6537.
13. Yoshikawa T., Kawamitsu H., Mitchell D.G., et. al.: ADC measurement of abdominal organs and lesions using parallel imaging technique. AJR Am J Roentgenol 2006; 187: pp. 522-1530.
14. Dose M.D., Zhong J., Gore J.C.: In vivo measurement of ADC change due to intravascular susceptibility variation. Magn Reson Med 1999; 41: pp. 236-240.
15. Zhong J., Kennan R.P., Fulbringht R.K., et. al.: Quantification of intravascular and extravascular contributions to bold effects induced by alteration in oxygenation or intravascular contrast agents. Magn Reson Med 1998; 40: pp. 526-536.
16. Yamada K., Kubota H., Kizu O., et. al.: Effect of intravenous gadolinium-DTPA on diffusion weighted images. Stroke 2002; 33: pp. 1799-1802.
17. Chen G., Jespersen S.N., Pedersen M., et. al.: Intravenous administration of Gd-DTPA prior to DWI does not affect the apparent diffusion constant. Magn Reson Imaging 2005; 23: pp. 685-689.