Rationale and Objectives
In this prospective study, we aimed to reveal the efficiency of phase-contrast magnetic resonance imaging (PC-MRI) in the diagnosis of idiopathic normal pressure hydrocephalus (INPH) and prediction of shunt response.
Materials and Methods
The study group consisted of 43 patients with INPH diagnosis and 15 asymptomatic age-matched controls. PC-MRI studies were applied on cerebral aqueduct and superior sagittal sinus (SSS) in all the cases.
Results
The maximum and mean cerebrospinal fluid (CSF) flow velocities were significantly higher in the INPH patients compared with the controls ( P < .05). CSF stroke volume (43.2 ± 63.8 μL) and output/min (3921 ± 5668 μL) were remarkably higher in the NPH group compared with the control group (3.9 ± 3.9 μL, 439 ± 487 μL, respectively) ( P < .05). Maximum and mean venous velocity values of the INPH patients (maximum, 19.2 ± 4.3 cm/s; mean, 16 ± 3.7 cm/s), were lower than those of the control group (maximum, 21.8 ± 4.6 cm/s; mean, 18.9 ± 3.9 cm/s) ( P < .05). Stroke volume and venous output/min values of INPH patients in SSS, were significantly lower than those of the control group ( P < .001, P = .007, respectively). The response of INPH patients against shunt treatment showed no statistical correlation with any of the PC-MRI parameters ( P > .05).
Conclusion
The measurement of CSF venous flow velocities with PC-MRI is a noninvasive test that benefits INPH diagnosis, but remains inadequate in prediction of response against shunt treatment.
Normal pressure hydrocephalus (NPH) demonstrates a normal initial pressure during lumbar puncture and follows a course including hydrocephalus and certain symptoms (dementia, gait disorder, urinary incontinence) . It is a rarer cause of cognitive deficits, responsible for 0%–5% of all dementia, but is probably being underestimated partly because of inconsistent definitions . Imaging reveals ventriculomegaly and flow void sign in the cerebral aqueduct without displaying a marked dilatation (differing from the one in cerebral atrophy) in cortical sulci . Because NPH is the only cause of dementia that benefits from shunt treatment, its differentiation from other dementia causes associated with ventriculomegaly bears importance . NPH may be either idiopathic (INPH) or secondary (SNPH) to subarachnoid hemorrhage, meningitis, cranial trauma, and intracranial surgery . The success rate of shunt treatment in NPH patients varies between 30% and 70%. INPH benefits less from shunt treatment (30%–50%) . Recently, most of the studies have been focused on determination of patients who would benefit from shunt treatment .
Many tests have been employed in the diagnosis of NPH before now. Among the imaging methods, we can mention radionuclide cisternography, computed tomography (CT), magnetic resonance (MRI), computed tomography cisternography, phase-contrast cine MRI (PC-MRI) . There is no test that can establish a definitive diagnosis or predict shunt response . PC-MRI is noted as a method that is helpful in NPH diagnosis and prediction of shunt therapy . In the present study, we aimed to determine the efficiency of PC-MRI in INPH diagnosis and prediction of treatment response.
Materials and methods
Study Group
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
MRI Study Protocol and Statistical Evaluation
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
CSF Flow Dynamics of NPH and Control Cases
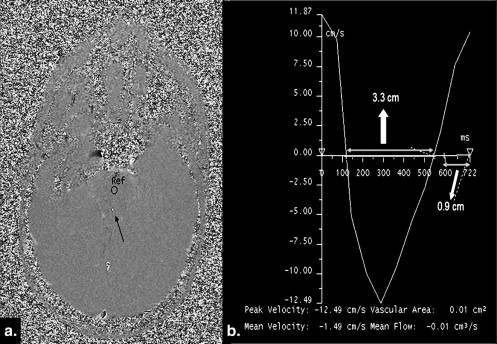
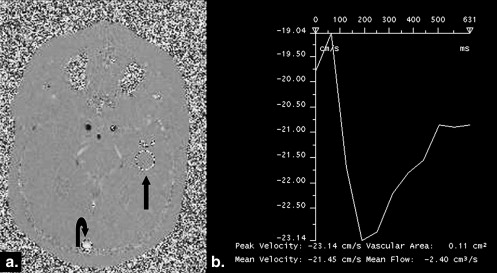
CSF parameters NPH cases (mean ± SD) Controls (mean ± SD) NPH vs. controls Shunt response Mean velocity 1.22 ± 1.54 cm/s 0.57 ± 0.36 cm/s_P_ < .05 NS Maximum velocity 8.53 ± 4.13 cm/s 4.78 ± 2.48 cm/s_P_ < .05 NS Stroke volume 43.2 ± 63.8 μL 3.9 ± 3.9 μlt_P_ < .001 NS Output/min 3921 ± 5668 μL 439 ± 487 μlt_P_ < .001 NS
CSF, cerebrospinal fluid; NS, not significant; SD, standard deviation.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 2
Venous Flow Dynamics of NPH and Control Cases
Venous parameters NPH cases (mean ± SD) Controls (mean ± SD) NPH vs. controls Shunt response Mean velocity 16 ± 3.7 cm/s 18.9 ± 3.9 cm/s_P_ < .05 NS Maximum velocity 19.2 ± 4.3 cm/s 21.8 ± 4.6 cm/s_P_ < .05 NS Stroke volume 1274 ± 741 μL 1828 ± 562 μl_P_ < .001 NS Output/min 141 ± 32 cm 3 195 ± 41 cm 3 P < .007 NS
NPH, normal pressure hydrocephalus; NS, not significant; SD, standard deviation.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
Acknowledgment
Get Radiology Tree app to read full this article<
References
1. Bateman G.A., Levi C.R., Schofield P., et. al.: The pathophysiology of the aqueduct stroke volume in normal pressure hydrocephalus: can co-morbidity with other forms of dementia be excluded?. Neuroradiology 2005; 47: pp. 741-748.
2. El Sankari S.S., Baledent O., Gondry-Jouet C., et. al.: Aging effects on cerebral blood and cerebrospinal fluid flows. J Cerebral Blood Flow Metab 2007; 27: pp. 1563-1572.
3. Kitagaki H., Mori E., Ishii K., et. al.: CSF spaces in idiopathic normal pressure hydrocephalus: Morphology and volumetry. AJNR Am J Neuroradiol 1998; 19: pp. 1277-1284.
4. Shiino A., Nishida Y., Yasuda H., et. al.: Magnetic resonance spectroscopic determination of a neuronal and axonal marker in white matter predicts reversibility of deficits in secondary normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 2004; 75: pp. 1141-1148.
5. Kahlon B., Annertz M., Ståhlberg F., et. al.: Is aqueductal stroke volume, measured with cine phase-contrast magnetic resonance imaging scans useful in predicting outcome of shunt surgery in suspected normal pressure hydrocephalus?. Neurosurgery 2007; 60: pp. 124-130.
6. Dixon G.R., Friedman J.A., Luetmer P.H., et. al.: Use of cerebrospinal fluid flow rates measured by phase-contrast MR to predict outcome of ventriculo-peritoneal shunting for idiopathic normal-pressure hydrocephalus. Mayo Clin Proc 2002; 77: pp. 509-514.
7. Bateman G.A.: The pathophysiology of idiopathic normal pressure hydrocephalus: cerebral ischemia or altered venous hemodynamics?. AJNR Am J Neuroradiol 2008; 1: pp. 198-203.
8. Brecknell J.E., Brown J.I.M.: Is idiopathic normal pressure hydrocephalus an independent entity?. Acta Neurochir 2004; 146: pp. 1003-1007.
9. Bradley W.G.: Cerebrospinal fluid dynamics and shunt responsiveness in patients with normal-pressure hydrocephalus. Mayo Clin Proc 2002; 77: pp. 507-508.
10. Bateman G.A.: Vascular compliance in normal pressure hydrocephalus. AJNR Am J Neuroradiol 2000; 21: pp. 1574-1585.
11. Ishikawa M.: Clinical guidelines for idiopathic normal pressure hydrocephalus. Neurol Med Chir 2004; 44: pp. 222-223.
12. Sasaki M., Honda S., Yuasa T., et. al.: Narrow CSF space at high convexity and high midline areas in idiopathic normal pressure hydrocephalus detected by axial and coronal MRI. Neuroradiology 2008; 50: pp. 117-122.
13. Bradley W.C., Scalzo D., Queralt J., et. al.: Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology 1996; 198: pp. 523-529.
14. Graff-Radford N.R.: Normal pressure hydrocephalus. Neurol Clin 2007; 25: pp. 809-832.
15. Scollato A., Tenenbaum R., Bahl G., et. al.: Changes in aqueductal CSF stroke volume and progression of symptoms in patients with unshunted idiopathic normal pressure hydrocephalus. AJNR Am J Neuroradiol 2008; 29: pp. 192-197.
16. Hebb A.O., Cusimano M.D.: Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery 2001; 49: pp. 1166-1184.
17. Kim D.S., Choi J.U., Huh R., et. al.: Quantitative assessment of cerebrospinal fluid hydrodynamics using a phase contrast cine MR image in hydrocephalus. Child’s Nerv Syst 1999; 15: pp. 461-467.
18. Greitz D.: Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg Rev 2004; 27: pp. 147-165.
19. Bradley W.G., Whittemore A.R., Kortman K.E., et. al.: Marked cerebrospinal fluid void: indicator of successful shunt in patients with suspected normal-pressure hydrocephalus. Radiology 1991; 178: pp. 459-466.
20. Wagshul M.E., Chen J.J., Egnor M.R., et. al.: Amplitude and phase of cerebrospinal fluid pulsations: experimental studies and review of literature. J Neurosurg 2006; 104: pp. 810-819.
21. Luetmer P.H., Huston J., Friedman J.A., et. al.: Measurement of cerebrospinal fluid flow at the cerebral aqueduct by use of phase-contrast magnetic resonance imaging: technique validation and utility in diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery 2002; 50: pp. 534-542.
22. Bateman G.A.: Magnetic resonance imaging quantification of compliance and collateral flow in late-onset idiopathic aqueductal stenosis: venous pathophysiology revisited. J Neurosurg 2007; 107: pp. 951-958.
23. Lee J.H., Lee H.K., Kim J.K., et. al.: CSF flow quantification of the cerebral aqueduct in normal volunteers using phase contrast cine MR imaging. Korean J Radiol 2004; 5: pp. 81-86.
24. De Marco G., Peretti , Poncelet A.D., et. al.: Intracranial fluid dynamics in normal and hydrocephalic states. Systems analysis with phase-contrast magnetic resonance imaging. J Comput Assist Tomogr 2004; 28: pp. 247-254.
25. Mase M., Yamada K., Banno T., et. al.: Quantitative analysis of CSF flow dynamics using MRI in normal pressure hydrocephalus. Acta Neurochir Suppl 1998; 71: pp. 350-353.
26. Baledent O., Gondry-Jouet C., Meyer M.E., et. al.: Relationship between cerebrospinal fluid and blood dynamics in healthy volunteers and patients with communicating hydrocephalus. Invest Radiol 2004; 39: pp. 45-55.
27. Bradley W.G.: Idiopathic normal pressure hydrocephalus: new findings and thoughts on etiology. AJNR Am J Neuroradiol 2008; 29: pp. 1-3.
28. Gideon P., Thomsen C., Gjerris F., et. al.: Measurement of blood flow in the superior sagittal sinus in healthy volunteers, and in patients with normal pressure hydrocephalus and idiopathic intracranial hypertension with phase-contrast cine MR imaging. Acta Radiol 1996; 37: pp. 171-176.
29. Rubenstein E.: Relationship of senescence of cerebrospinal fluid circulatory system to dementias of the aged. Lancet 1998; 351: pp. 283-285.
30. Yldz H., Erdoğan C., Yalçn R., et. al.: Evaluation of communication between intracranial arachnoid cysts and cisterns with phase-contrast cine MR imaging. AJNR Am J Neuroradiol 2005; 26: pp. 145-151.
31. Algin O., Hakyemez B., Gokalp G., et. al.: Phase-contrast cine MRI versus MR cisternography on the evaluation of the communication between intraventricular arachnoid cysts and neighboring cerebrospinal fluid spaces. Neuroradiology 2009; 51: pp. 305-312.