The technology behind positron emission tomography (PET) and the most widely used tracer, 2-deoxy-2-[18F]fluoro-D-glucose (FDG), were both conceived in the 1970s, but the latest decade has witnessed a rapid emergence of FDG-PET as an effective imaging technique. This is not least due to the emergence of hybrid scanners combining PET with computed tomography (PET/CT). Molecular imaging has enormous potential for advancing biological research and patient care, and FDG-PET/CT is currently the most widely used technology in this domain. In this review, we discuss contemporary applications of FDG-PET and FDG-PET/CT as well as novel developments in quantification and potential future indications including the emerging new modality PET/magnetic resonance imaging.
Although the technology behind positron emission tomography (PET) and the most widely used tracer, 2-deoxy-2-[ 18 F]fluoro-D-glucose (FDG), were both conceived in the 1970s ( Figure 1 ) , the latest decade has witnessed a rapid emergence of FDG-PET as an effective imaging technique. This is not least due to the appearance of hybrid scanners combining PET with computed tomography (PET/CT), the gradual approval of these novel techniques by the US Food and Drug Administration (FDA), and subsequent reimbursement coverage by the Center of Medicare and Medicaid Services (CMS) in the United States. Molecular imaging has enormous potential for advancing biological research and patient care, and FDG-PET/CT is currently the most widely used technology in this domain.
Glucose and glucose analogs enter any cell via a family of ubiquitous transmembrane transporters called glucose transporters (GLUTs). On entry into the cell, they can produce energy in the form of adenosine triphosphate by way of the glycolytic pathway regardless of the presence of molecular oxygen in the cell . Many malignant cells are hypermetabolic and highly glycolytic, a knowledge dating back to the 1930s when Warburg demonstrated increased glucose metabolism in malignant cells in vitro . To facilitate this high level of glycolysis, many different cancer cells have been shown to be characterized by an upregulation of GLUT and an increased expression of glycolytic enzymes and hexokinase activity, although not ubiquitously present in all cancers . The transport of glucose into cells is facilitated by a downhill gradient maintained by the phosphorylation of glucose to glucose-6-phosphate by hexokinases. This mechanism also applies to FDG, which is converted to FDG-6-phosphate once it enters the cell. However, because of a subtle, but significant, stereochemical difference, FDG-6-phosphate is not a substrate for further metabolism in the glycolytic pathway. Neither glucose-6-phosphate nor FDG-6-phosphate can leave the cell by way of GLUT. Instead, the effect of hexokinase is reversed by glucose-6-phosphatase, which dephosphorylates glucose or FDG, enabling it to leave the cell. However, tumor cells contain low levels of glucose-6-phosphatase to counteract hexokinases, and as such, FDG-6-phosphate is metabolically trapped inside the cell. Combined with the increased rates of glycolysis, the result is a net accumulation of FDG in many malignant cells relative to normal cells, making FDG an excellent and sensitive marker for changes in glucose metabolism . The degree of FDG retention is dictated by the concentration and degree of activity of glucose-6-phosphatase. This is important because these factors may vary considerably between highly differentiated and undifferentiated tumor cells, which will result in producing varying degrees of uptake and retention profiles over time for this tracer. There are also differences in the enzyme concentration and enzyme activity in benign and malignant diseases with a more rapid cellular washout of FDG in normal tissues and in benign disorders because of higher levels of glucose-6-phosphatase . This observation may be used advantageously to differentiate between benign inflammatory cells and malignant ones. This can be achieved by multiple time point imaging, which will reveal different uptake patterns over time between the two scans. Delayed imaging can also be of great value for visualizing highly differentiated cancers, which may have limited FDG avidity initially but become positive during later scans.
FDG-PET and FDG-PET/CT are not without limitations. Stand-alone PET lacks spatial resolution and anatomic correlation, but this is alleviated by the hybrid PET/CT technology. Furthermore, FDG is a nonspecific tracer. This is especially challenging in the overall differentiating between the main clinical indications, malignancies and inflammatory conditions, but also within these disease entities, there may be issues caused by the nonspecific changes in uptake following instrumentation, surgery, chemotherapy, and radiotherapy. However, there are also intrinsic factors affecting the specificity. First, GLUT transporters are ubiquitous, and physiologic uptake may be present in many organs or tissues using glucose, and thus, there are many potential sites of nonspecific physiologic FDG uptake, most notably muscles, brain, and heart. FDG is excreted through urine, which may hamper the assessment of the urinary tract. FDG is also excreted to the bowel, and furthermore, the normal bacterial flora uses glucose to some extent, which may give rise to both focal and diffuse uptake in the bowel. Some advocate catheter a demeure and bowel cleansing, but there is no consensus on the routine use. Finally, FDG competes with glucose and thus the net FDG uptake in pathologic processes depends on blood glucose levels .
With this in mind, some basic considerations in patient preparation before FDG injection should be considered. Skeletal muscle is by far the largest organ structure and accounts for the majority of glucose metabolism in healthy human beings. Therefore, it also has the largest FDG uptake capacity . The degree of physiologic FDG uptake should therefore be decreased as much as possible to minimize any detrimental effect on target-to-background contrast, which may hamper the detection of pathologically FDG-avid lesions. This is accomplished by fasting for at least 4–6 hours before FDG injection, avoiding exercise, and resting during the uptake period .
The plasma glucose level in patients should also be normal before the administration of FDG. Skeletal muscles mostly take up FDG by insulin stimulation of GLUT, and euglycemic hyperinsulinemia has been shown experimentally to cause increased FDG uptake in muscles with no effect on malignant cells . Despite sufficient fasting, chronic hyperglycemia (>200 mg/dL [∼11 mmol/L]) is not uncommon as it occurs often in patients with type 2 diabetes. However, this has been shown to have minimal effects on the diagnostic performance of FDG-PET with no correlation between FDG muscle uptake and plasma glucose level . Therefore, if scans cannot be rescheduled, hyperglycemia is preferable to hyperinsulinemia, and administering insulin before FDG injection should be avoided. Of course, these caveats become even more important if FDG is to be used for assessing the musculoskeletal system, for example, evaluation of potential skeletal metastases.
Get Radiology Tree app to read full this article<
FDG-PET in central nervous system disorders
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
FDG-PET in malignancies
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
FDG-PET in atherosclerosis and myocardial viability
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
FDG-PET in inflammation processes
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
FDG-PET in decades to come
Quantifying Disease
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Hybrid PET/MRI
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Epilogue
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Alavi A., Reivich M.: The conception of FDG-PET imaging. Semin Nucl Med 2002; 32: pp. 2-5.
2. Vallabhajosula S.: 18 F-labeled positron emission tomographic radiopharmaceuticals in oncology: An overview of radiochemistry and mechanisms of tumor localization. Semin Nucl Med 2007; 37: pp. 400-419.
3. Warburg O.: On the origin of cancer cells. The metabolism of tumors.1931.Richard R. SmithNew Yorkpp. 129-169.
4. Macheda M.L., Rogers S., Best J.D.: Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol 2005; 202: pp. 654-662.
5. Medina R.A., Owen G.I.: Glucose transporters: expression, regulation and cancer. Biol Res 2002; 35: pp. 9-26.
6. Basu S., Kung J., Houseni M., et. al.: Temporal profile of fluorodeoxyglucose uptake in malignant lesions and normal organs over extended time periods in patients with lung carcinoma: implications for its utilization in assessing malignant lesions. Q J Nucl Med Mol Imaging 2009; 53: pp. 9-19.
7. Kapoor V., McCook B.M., Torok F.S.: An introduction to PET-CT imaging. Radiographics 2004; 24: pp. 523-543.
8. Reinhardt M., Beu M., Vosberg H., et. al.: Quantification of glucose transport and phosphorylation in human skeletal muscle using FDG PET. J Nucl Med 1999; 40: pp. 977-985.
9. Lindholm H., Johansson O., Jonsson C., et. al.: The distribution of FDG at PET examinations constitutes a relative mechanism: significant effects at activity quantification in patients with a high muscular uptake. Eur J Nucl Med Mol Imaging 2012; 39: pp. 1685-1690.
10. Minn H., Nuutila P., Lindholm P., et. al.: In vivo effects of insulin on tumor and skeletal muscle glucose metabolism in patients with lymphoma. Cancer 1994; 73: pp. 1490-1498.
11. Hara T., Higashi T., Nakamoto Y., et. al.: Significance of chronic marked hyperglycemia on FDG-PET: is it really problematic for clinical oncologic imaging?. Ann Nucl Med 2009; 23: pp. 657-669.
12. Herholz K., Langen K.J., Schiepers C., et. al.: Brain tumors. Semin Nucl Med 2012; 42: pp. 356-370.
13. Willmann O., Wennberg R., May T., et. al.: The contribution of 18F-FDG PET in preoperative epilepsy surgery evaluation for patients with temporal lobe epilepsy: A meta-analysis. Seizure 2007; 16: pp. 509-520.
14. Bloudek L.M., Spackman E., Blankenburg M., et. al.: Review and meta-analysis of biomarkers and diagnostic imaging in Alzheimer’s disease. J Alzheimers Dis 2011; 26: pp. 627-645.
15. Moghbel M.C., Saboury B., Basu S., et. al.: Amyloid-β imaging with PET in Alzheimer’s disease: is it feasible with current radiotracers and technologies?. Eur J Nucl Med Mol Imaging 2012; 39: pp. 202-208.
16. Kepe V., Moghbel M.C., Långström B., et. al.: Amyloid-β positron emission tomography imaging probes: a critical review. J Alzheimers Dis 2013 May 6; [Epub ahead of print]
17. Zhang S., Han D., Tan X., et. al.: Diagnostic accuracy of 18 F-FDG and 11 C-PIB-PET for prediction of short-term conversion to Alzheimer’s disease in subjects with mild cognitive impairment. Int J Clin Pract 2012; 66: pp. 185-198.
18. Brooks D.J.: Parkinson’s disease: diagnosis. Parkinsonism Relat Disord 2012; 18: pp. 31-33.
19. Newberg A.B., Moss A.S., Monti D.A., et. al.: Positron emission tomography in psychiatric disorders. Ann N Y Acad Sci 2011; 1228: pp. 13-25.
20. Som P., Atkins H.L., Bandoypadhyay D., et. al.: A fluorinated glucose analog, 2-fluoro-2-deoxy-D-glucose (F-18): nontoxic tracer for rapid tumor detection. J Nucl Med 1980; 21: pp. 670-675.
21. Yonekura Y., Benua R.S., Brill A.B., et. al.: Increased accumulation of 2-deoxy-2-[18F]Fluoro-D-glucose in liver metastases from colon carcinoma. J Nucl Med 1982; 23: pp. 1133-1137.
22. Alavi J.B., Alavi A., Goldberg H.I., et. al.: Sequential computerized tomography and positron emission tomography studies in a patient with malignant glioma. Nucl Med Commun 1987; 8: pp. 457-468.
23. DiChiro G., Brooks R.A., Sokoloff L., et. al.: Glycolytic rate and histologic grade of human cerebral gliomas: A study with [18F]fluorodeoxyglucose and positron emission tomography. Positron Emission Tomography of the Brain 1983; pp. 181-191.
24. Gambhir S.S., Czernin J., Schwimmer J., et. al.: A tabulated summary of the FDG PET literature. J Nucl Med 2001; 42: pp. 1S-93S.
25. Facey K., Bradbury I., Laking G., et. al.: Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess 2007; 11: iii-iv, xi–267
26. Czernin J., Allen-Auerbach M., Schelbert H.R.: Improvements in cancer staging with PET/CT: literature-based evidence as of September 2006. J Nucl Med 2007; 48: pp. 78S-88S.
27. Bockisch A., Freudenberg L.S., Schmidt D., et. al.: Hybrid imaging by SPECT/CT and PET/CT: proven outcomes in cancer imaging. Semin Nucl Med 2009; 39: pp. 276-289.
28. Histed S.N., Lindenberg M.L., Mena E., et. al.: Review of functional anatomical imaging in oncology. Nucl Med Comm 2012; 33: pp. 349-361.
29. Basu S., Alavi A.: Unparalleled contribution of 18F-FDG PET to medicine over 3 decades. J Nucl Med 2008; 49: 17N–21N,3N
30. Basu S.: The scope and potentials of functional radionuclide imaging towards advancing personalized medicine in oncology: Emphasis on PET-CT. Discov Med 2012; 13: pp. 65-73.
31. Høilund-Carlsen P.F., Gerke O., Vilstrup M.H., et. al.: PET/CT without capacity limitations: a Danish experience from a European perspective. Eur Radiol 2011; 21: pp. 1277-1285.
32. Pavlidis N., Fizazi K.: Carcinoma of unknown origin (CUP). Crit Rev Oncol Hematol 2009; 69: pp. 271-278.
33. Kwee T.C., Kwee R.M.: Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Radiol 2009; 19: pp. 731-744.
34. Linke R., Schroeder M., Helmberger T., et. al.: Antibody-positive paraneoplastic neurologic syndromes: value of CT and PET for tumor diagnosis. Neurology 2004; 63: pp. 282-286.
35. Schramm N., Rominger A., Schmidt C., et. al.: Detection of underlying malignancy in patients with paraneoplastic neurological syndromes: comparison of (18)F-FDG PET/CT and contrast-enhanced CT. Eur J Nucl Med Mol Imaging 2013; 40: pp. 1014-1024.
36. Basu S., Alavi A.: Role of FDG-PET in the clinical management of paraneoplastic neurological syndrome: Detection of the underlying malignancy and the brain PET-MRI correlates. Mol Imaging Biol 2008; 10: pp. 131-137.
37. Bannas P., Weber C., Derlin T., et. al.: 18F-FDG-PET/CT in the diagnosis of paraneoplastic neurological syndromes: a retrospective analysis. Eur Radiol 2010; 20: pp. 923-930.
38. Vaidyanathan S., Pennington C., Ng C.Y., et. al.: 18F-FDG-PET-CT in the evaluation of paraneoplastic syndromes: experience at a regional oncology centre. Nucl Med Commun 2012; 33: pp. 872-880.
39. Selva-O’Callaghan A., Grau J.M., Gámez-Cenzano C., et. al.: Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med 2010; 123: pp. 558-562.
40. Kyle R.A., Rajkumar S.V.: Multiple myeloma. Blood 2008; 111: pp. 2962-2972.
41. Hanrahan C.J., Christensen C.R., Crim J.R.: Current concepts in the evaluation of multiple myeloma with MR imaging and FDG PET/CT. Radiographics 2010; 30: pp. 127-142.
42. Zamagni E., Cavo M.: The role of imaging techniques in the management of multiple myeloma. Br J Haematol 2012; 159: pp. 499-513.
43. Lütje S., de Rooy J.W.J., Croockewit S., et. al.: Role of radiography, MRI and FDG-PET/CT in diagnosing, staging and therapeutical evaluation of patients with multiple myeloma. Ann Hematol 2009; 88: pp. 1161-1168.
44. Shie P., Cardarelli R., Sprawls K., et. al.: Systematic review: prevalence of malignant incidental thyroid nodules identified on fluorine-18 fluorodeoxyglucose positron emission tomography. Nucl Med Commun 2009; 30: pp. 742-748.
45. Chae E.Y., Cha J.H., Kim H.H., et. al.: Analysis of incidental focal hypermetabolic uptake in the breast as detected by 18F-FDG PET/CT: clinical significance and differential diagnosis. Acta Radiol 2012; 53: pp. 530-535.
46. Treglia G., Calcagni M.L., Rufini V., et. al.: Clinical significance of incidental focal colorectal 18F-fluorodeoxyglucose uptake: our experience and a review of the literature. Colorectal Dis 2011; 14: pp. 174-180.
47. Beatty J.S., Williams H.T., Aldridge B.A., et. al.: Incidental PET/CT findings in the cancer patient: how should they be managed?. Surgery 2009; 146: pp. 274-281.
48. Kwee T.C., Basu S., Saboury B., et. al.: A new dimension of FDG-PET interpretation: assessment of tumor biology. Eur J Nucl Med Mol Imaging 2011; 38: pp. 1158-1170.
49. Hofman M.S., Hicks R.: Changing paradigms with molecular imaging of neuroendocrine tumors. Discov Med 2012; 14: pp. 71-81.
50. Vach W., Høilund-Carlsen P.F., Gerke O., et. al.: Generating evidence for clinical benefit of PET/CT in diagnosing cancer patients. J Nucl Med 2011; 52: pp. 77S-85S.
51. Tawakol A., Fayad Z.A., Mogg R., et. al.: Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: Results of a multi-center FDG-PET/CT feasibility study. J Am Coll Cardiol 2013;
52. Tahara N., Kai H., Ishibashi M., et. al.: Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 2006; 48: pp. 1825-1831.
53. Rudd J.H., Warburton E.A., Fryer T.D., et. al.: Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 2002; 105: pp. 2708-2711.
54. Folco E.J., Sheikine Y., Rocha V.Z., et. al.: Hypoxia but not inflammation augments glucose uptake in human macrophages: Implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-D-glucose positron emission tomography. J Am Coll Cardiol 2011; 58: pp. 603-614.
55. Tawakol A., Migrino R.Q., Bashian G.G., et. al.: In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006; 48: pp. 1818-1824.
56. Marnane M., Merwick A., Sheehan O.C., et. al.: Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann Neurol 2012; 71: pp. 709-718.
57. Yun M., Jang S., Cucchiara A., et. al.: 18F FDG uptake in the large arteries: a correlation study with the atherogenic risk factors. Semin Nucl Med 2002; 32: pp. 70-76.
58. Bucerius J., Mani V., Moncrieff C., et. al.: Impact of noninsulin-dependent type 2 diabetes on carotid wall 18F-fluorodeoxyglucose positron emission tomography uptake. J Am Coll Cardiol 2012; 59: pp. 2080-2088.
59. Yun M., Yeh D., Araujo L.I., et. al.: F-18 FDG uptake in the large arteries: a new observation. Clin Nucl Med 2001; 26: pp. 314-319.
60. Tatsumi M., Cohade C., Nakamoto Y., et. al.: Fluorodeoxyglucose uptake in the aortic wall at PET/CT: possible finding for active atherosclerosis. Radiology 2003; 229: pp. 831-837.
61. Bucerius J., Duivenvoorden R., Mani V., et. al.: Prevalence and risk factors of carotid vessel wall inflammation in coronary artery disease patients: FDG-PET and CT imaging study. JACC Cardiovasc Imaging 2011; 4: pp. 1195-1205.
62. Kim T.N., Kim S., Yang S.J., et. al.: Vascular inflammation in patients with impaired glucose tolerance and type 2 diabetes: analysis with 18F-fluorodeoxyglucose positron emission tomography. Circ Cardiovasc Imaging 2010; 3: pp. 142-148.
63. Tahara N., Kai H., Yamagishi S., et. al.: Vascular inflammation evaluated by [18F]-fluorodeoxyglucose positron emission tomography is associated with the metabolic syndrome. J Am Coll Cardiol 2007; 49: pp. 1533-1539.
64. Davies J.R., Rudd J.H., Fryer T.D., et. al.: Identification of culprit lesions after transient ischemic attack by combined 18F fluorodeoxyglucose positron-emission tomography and high-resolution magnetic resonance imaging. Stroke 2005; 36: pp. 2642-2647.
65. Paulmier B., Duet M., Khayat R., et. al.: Arterial wall uptake of fluorodeoxyglucose on PET imaging in stable cancer disease patients indicates higher risk for cardiovascular events. J Nucl Cardiol 2008; 15: pp. 209-217.
66. Rominger A., Saam T., Wolpers S., et. al.: 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med 2009; 50: pp. 1611-1620.
67. Tahara N., Kai H., Ishibashi M., et. al.: Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 2006; 48: pp. 1825-1831.
68. Ishii H., Nishio M., Takahashi H., et. al.: Comparison of atorvastatin 5 and 20 mg/d for reducing F-18 fluorodeoxyglucose uptake in atherosclerotic plaques on positron emission tomography/computed tomography: a randomized, investigator-blinded, open-label, 6-month study in Japanese adults scheduled for percutaneous coronary intervention. Clin Ther 2010; 32: pp. 2337-2347.
69. Lee S.J., On Y.K., Lee E.J., et. al.: Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med 2008; 49: pp. 1277-1282.
70. Duivenvoorden R., Fayad Z.A.: Utility of atherosclerosis imaging in the evaluation of high-density lipoprotein-raising therapies. Curr Atheroscler Rep 2011; 13: pp. 277-284.
71. Font M.A., Fernandez A., Carvajal A., et. al.: Imaging of early inflammation in low-to-moderate carotid stenosis by 18-FDG-PET. Front Biosci 2009; 14: pp. 3352-3360.
72. Rudd J.H., Myers K.S., Bansilal S., et. al.: Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med 2008; 49: pp. 871-878.
73. Rudd J.H., Myers K.S., Bansilal S., et. al.: (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol 2007; 50: pp. 892-896.
74. Camici P.G., Prasad S.K., Rimoldi O.E.: Stunning, hibernation, and assessment of myocardial viability. Circulation 2008; 117: pp. 103-114.
75. Tillisch J., Brunken R., Marshall R., et. al.: Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med 1986; 314: pp. 884-888.
76. Auerbach M.A., Schoder H., Hoh C., et. al.: Prevalence of myocardial viability as detected by positron emission tomography in patients with ischemic cardiomyopathy. Circulation 1999; 99: pp. 2921-2926.
77. Schinkel A.F., Bax J.J., Poldermans D., et. al.: Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol 2007; 32: pp. 375-410.
78. Schinkel A.F., Bax J.J., Delgado V., et. al.: Clinical relevance of hibernating myocardium in ischemic left ventricular dysfunction. Am J Med 2010; 123: pp. 978-986.
79. Gaemperli O., Kaufmann P.A.: PET and PET/CT in cardiovascular disease. Ann N Y Acad Sci 2011; 1228: pp. 109-136.
80. Basu S., Zhuang H., Torigian D.A., et. al.: Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin Nucl Med 2009; 39: pp. 124-145.
81. Basu S., Chryssikos T., Moghadam-Kia S., et. al.: Positron emission tomography as a diagnostic tool in infection: present role and future possibilities. Semin Nucl Med 2009; 39: pp. 36-51.
82. Zhuang H., Yu J.Q., Alavi A.: Applications of fluorodeoxyglucose-PET imaging in the detection of infection and inflammation and other benign disorders. Radiol Clin North Am 2005; 43: pp. 121-134.
83. Kumar R., Basu S., Torigian D., et. al.: Role of modern imaging techniques for diagnosis of infection in the era of 18F-fluorodeoxyglucose positron emission tomography. Clin Microbiol Rev 2008; 21: pp. 209-224.
84. Basu S., Chryssikos T., Moghadam-Kia S., et. al.: Positron emission tomography as a diagnostic tool in infection: present role and future possibilities. Semin Nucl Med 2009; 39: pp. 36-51.
85. Tahara T., Ichiya Y., Kuwabara Y., et. al.: High [18F]-fluorodeoxyglucose uptake in abdominal abscesses: a PET study. J Comput Assist Tomogr 1989; 13: pp. 829-831.
86. Knockaert D.C., Vanderschueren S., Blockmans D.: Fever of unknown origin in adults: 40 years on. J Intern Med 2003; 253: pp. 263-275.
87. Israel O., Keidar Z.: PET/CT imaging in infectious conditions. Ann N Y Acad Sci 2011; 1228: pp. 150-166.
88. Kluge S., Braune S., Nierhaus A., et. al.: Diagnostic value of positron emission tomography combined with computed tomography for evaluating patients with septic shock of unknown origin. J Critical Care 2012; 27: pp. 316.e1-316.e7.
89. Bleeker-Rovers C.P., Vos F.J., Wanten G.J.A., et. al.: 18F-FDG PET in detecting metastatic infectious disease. J Nucl Med 2005; 46: pp. 2014-2019.
90. Hess S., Vind S.H., Skarphédinsson S., et. al.: Clinical value of PET/CT in bacteraemia of unknown origin. Results from an observational pilot study. Eur J Nucl Med Mol Imaging 2010; 37: pp. S468.
91. Vos F.J., Bleeker-Rovers C.P., Sturm P.D., et. al.: 18F-FDG PET/CT for detection of metastatic infection in gram-positive bacteremia. J Nucl Med 2010; 51: pp. 1234-1240.
92. Vos F.J., Bleeker-Rovers C.P., Kullberg B.J., et. al.: Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med 2011; 52: pp. 1673-1678.
93. Millar B.C., Prendergast B.D., Alavi A., et. al.: (18)FDG-positron emission tomography (PET) has a role to play in the diagnosis and therapy of infective endocarditis and cardiac device infection. Int J Cardiol 2013 Jan 10; doi:pii: S0167–5273(12)01633-6. 10.1016/j.ijcard.2012.12.005. [Epub ahead of print]
94. Alavi A., Saboury B., Basu S.: Imaging the infected heart. Sci Transl Med 2011; 3: 99fs3
95. Guy S.D., Tramontana A.R., Worth L.J., et. al.: Use of FDG PET/CT for investigation of febrile neutropenia: evaluation in high-risk cancer patients. Eur J Nucl Med Mol Imaging 2012; 39: pp. 1348-1355.
96. Vos F.J., Donnelly J.P., Oyen W.J.G., et. al.: 18-F-FDG PET/CT for diagnosing infectious complications in patients with severe neutropenia after intensive chemotherapy for haematological malignancy or stem cell transplantation. Eur J Nucl Med Mol Imaging 2012; 39: pp. 120-128.
97. O’Doherty M.J., Barrington S.F., Campbell M., et. al.: PET scanning and the human immunodeficiency virus-positive patient. J Nucl Med 1997; 38: pp. 1575-1583.
98. Castaigne C., Tondeur M., de Wit S., et. al.: Clinical value of FDG PET/CT for the diagnosis of human immunodeficiency virus-associated fever of unknown origin: a retrospective study. Nucl Med Commun 2009; 30: pp. 41-47.
99. Termaat M.F., Raijmakers P.G., Scholten H.J., et. al.: The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: a systematic review and meta-analysis. J Bone Joint Surg Am 2005; 87: pp. 2464-2471.
100. Schmitz A., Risse J.H., Grünwald F., et. al.: Fluorine-18 fluorodeoxyglucose positron emission tomography findings in spondylodiscitis: preliminary results. Eur Spine J 2001; 10: pp. 534-539.
101. Stumpe K.D., Zanetti M., Weishaupt D., et. al.: FDG positron emission tomography for differentiation of degenerative and infectious endplate abnormalities in the lumbar spine detected on MR imaging. AJR Am J Roentgenol 2002; 179: pp. 1151-1157.
102. De Winter F., Gemmel F., Van De Wiele C., et. al.: 18-Fluorine fluorodeoxyglucose positron emission tomography for the diagnosis of infection in the postoperative spine. Spine (Phila Pa 1976) 2003; 28: pp. 1314-1319.
103. Schiesser M., Stumpe K.D., Trentz O., et. al.: Detection of metallic implant-associated infections with FDG PET in patients with trauma: correlation with microbiologic results. Radiology 2003; 226: pp. 391-398.
104. Hartmann A., Eid K., Dora C., et. al.: Diagnostic value of 18F-FDG PET/CT in trauma patients with suspected chronic osteomyelitis. Eur J Nucl Med Mol Imaging 2007; 34: pp. 704-714.
105. Kwee T.C., Kwee R.M., Alavi A.: FDG-PET for diagnosing prosthetic joint infection: systematic review and metaanalysis. Eur J Nucl Med Mol Imaging 2008; 35: pp. 2122-2132.
106. Kwee T.C., Basu S., Torigian D.A., et. al.: FDG PET imaging for diagnosing prosthetic joint infection: discussing the facts, rectifying the unsupported claims and call for evidence-based and scientific approach. Eur J Nucl Med Mol Imaging 2013; 40: pp. 464-466.
107. Nawaz A., Torigian D.A., Siegelman E.S., et. al.: Diagnostic performance of FDG-PET, MRI, and plain film radiography (PFR) for the diagnosis of osteomyelitis in the diabetic foot. Mol Imaging Biol 2010; 12: pp. 335-342.
108. Basu S., Chryssikos T., Houseni M., et. al.: Potential role of FDG PET in the setting of diabetic neuro-osteoarthropathy: can it differentiate uncomplicated Charcot’s neuroarthropathy from osteomyelitis and soft-tissue infection?. Nucl Med Commun 2007; 28: pp. 465-472.
109. Kagna O., Srour S., Melamed E., et. al.: FDG PET/CT imaging in the diagnosis of osteomyelitis in the diabetic foot. Eur J Nucl Med Mol Imaging 2012; 39: pp. 1545-1550.
110. Legout L., D’Elia P.V., Sarraz-Bourmet B., et. al.: Diagnosis and management of prosthetic vascular graft infections. Med Mal Infect 2012; 42: pp. 102-109.
111. Basu S., Alavi A.: Emerging role of FDG-PET for optimal response assessment in infectious diseases and disorders. Expert Rev Anti Infect Ther 2011; 9: pp. 143-145.
112. Nanni C., Boriani L., Salvadori C., et. al.: FDG PET/CT is useful for the interim evaluation of response to therapy in patients affected by haematogenous spondylodiscitis. Eur J Nucl Med Mol Imaging 2012; 39: pp. 1538-1544.
113. Zerizer I., Tan K., Khan S., et. al.: Role of FDG-PET and PET/CT in the diagnosis and management of vasculitis. Eur J Radiol 2010; 73: pp. 504-509.
114. Yamashita H., Kubota K., Takahashi Y., et. al.: Whole-body fluorodeoxyglucose positron emission tomography/computed tomography in patients with active polymyalgia rheumatica: evidence for distinctive bursitis and large-vessel vasculitis. Mod Rheumatol 2012; 22: pp. 705-711.
115. Basu S., Zhuang H., Torigian D.A., et. al.: Functional imaging of inflammatory diseases using nuclear medicine techniques. Sem Nucl Med 2009; 39: pp. 124-145.
116. Alavi A., Buchpiquel C.A., Loessner A.: Is there a role for FDG PET imaging in the management of patients with sarcoidosis?. J Nucl Med 1994; 35: pp. 1650-1652.
117. Kwee T.C., Torigian D.A., Alavi A.: Nononcological applications of positron emission tomography for evaluation of the thorax. J Thorac Imaging 2013; 28: pp. 25-39.
118. Israel-Biet D., Valeyre D.: Diagnosis of pulmonary sarcoidosis. Curr Opin Pulm Med 2013 Jul 22; [Epub ahead of print]
119. Treglia G., Taralli S., Giordano A.: Emerging role of whole-body 18F-fluorodeoxyglucose positron emission tomography as a marker of disease activity in patients with sarcoidosis: a systematic review. Sarcoidosis Vasc Diffuse Lung Dis 2011; 28: pp. 87-94.
120. Treglia G., Quartuccio N., Sadeghi R., et. al.: Diagnostic performance of Fluorine-18-Fluorodeoxyglucose positron emission tomography in patients with chronic inflammatory bowel disease: a systematic review and a meta-analysis. J Crohns Colitis 2013; 7: pp. 345-354.
121. Holtmann M.H., Uenzen M., Helisch A., et. al.: (18)F-fluorodeoxyglucose positron-emission tomography (PET) can be used to assess inflammation non-invasively in Crohn’s disease. Dig Dis Sci 2012; 57: pp. 2658-2668.
122. Ahmadi A., Li Q., Muller K., et. al.: Diagnostic value of noninvasive combined fluorine-18 labeled fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography enterography in active Crohn’s disease. Inflamm Bowel Dis 2010; 16: pp. 974-981.
123. Däbritz J., Jasper N., Loeffler M., et. al.: Noninvasive assessment of pediatric inflammatory bowel disease with 18F-fluorodeoxyglucose-positron emission tomography and computed tomography. Eur J Gastroenterol Hepatol 2011; 23: pp. 81-89.
124. Lenze F., Wessling J., Bremer J., et. al.: Detection and differentiation of inflammatory versus fibromatous Crohn’s disease strictures: prospective comparison of (18) F-FDG-PET/CT, MR-enteroclysis, and transabdominal ultrasound versus endoscopic/histologic evaluation. Inflamm Bowel Dis 2012; 18: pp. 2252-2260.
125. Carey K., Saboury B., Basu S., et. al.: Evolving role of FDG PET imaging in assessing joint disorders: a systematic review. Eur J Nucl Med Mol Imaging 2011; 38: pp. 1939-1955.
126. dos Anjos D.A., do Vale G.F., Campos C de M., et. al.: Extra-articular inflammatory sites detected by F-18 FDG PET/CT in a patient with rheumatoid arthritis. Clin Nucl Med 2010; 35: pp. 540-541.
127. Strobel K., von Hochstetter A.R., Exner U.G.: FDG uptake in a rheumatoid nodule with imaging appearance similar to a malignant soft tissue tumor. Clin Nucl Med 2009; 34: pp. 691-692.
128. Mountz J.M., Alavi A., Mountz J.D.: Emerging optical and nuclear medicine imaging methods in rheumatoid arthritis. Nat Rev Rheumatol 2012; 8: pp. 719-728.
129. Kubota K., Ito K., Morooka M., et. al.: FDG PET for rheumatoid arthritis: basic considerations and whole-body PET/CT. Ann N Y Acad Sci 2011; 1228: pp. 29-38.
130. Sarma M., Vijayant V., Basu S.: (18)F-FDG-PET assessment of early treatment response of articular and extra-articular foci in newly diagnosed rheumatoid arthritis. Hell J Nucl Med 2012; 15: pp. 70-71.
131. Elzinga E.H., van der Laken C.J., Comans E.F., et. al.: 18F-FDG PET as a tool to predict the clinical outcome of infliximab treatment of rheumatoid arthritis: an explorative study. J Nucl Med 2011; 52: pp. 77-80.
132. Aliyev A., Saboury B., Kwee T.C., et. al.: Age-related inflammatory changes in the spine as demonstrated by (18)F-FDG-PET: observation and insight into degenerative spinal changes. Hell J Nucl Med 2012; 15: pp. 197-201.
133. Lotz J.C., Haughton V., Boden S.D., et. al.: New treatments and imaging strategies in degenerative disease of the intervertebral disks. Radiology 2012; 264: pp. 6-19.
134. Mehta N.N., Yu Y., Saboury B., et. al.: Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol 2011; 147: pp. 1031-1039.
135. Yu Y., Sheth N., Krishnamoorthy P., et. al.: Aortic vascular inflammation in psoriasis is associated with HDL particle size and concentration: a pilot study. Am J Cardiovasc Dis 2012; 2: pp. 285-292.
136. Hess S., Madsen P.H., Basu S., et. al.: FDG PET/CT in venous thromboembolic disease. Clin Nucl Med 2012; 37: pp. 1170-1172.
137. Rondina M.T., Lam U.T., Pendleton R.C., et. al.: F-18 fluorodeoxyglucose positron emission tomography in the evaluation of acuity of deep vein thrombosis. Clin Nucl Med 2012; 37: pp. 1139-1145.
138. Adams M.C., Turkington T.G., Wilson J.M., et. al.: A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol 2010; 195: pp. 310-320.
139. Boellaard R., O’Doherty M.J., Weber W.A., et. al.: FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging 2010; 37: pp. 181-200.
140. Wahl R.L., Jacene H., Kasamon Y., et. al.: From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 2009; 50: pp. 122s-150s.
141. Soret M., Bacharach S.L., Buvat I.: Partial-volume effect in PET tumor imaging. J Nucl Med 2007; 48: pp. 932-945.
142. Chawluk J.B., Alavi A., Dann R., et. al.: Positron emission tomography in aging and dementia: effect of cerebral atrophy. J Nucl Med 1987; 28: pp. 431-437.
143. Soret M., Bacharach S.L., Buvat I.: Partial-volume effect in PET tumor imaging. J Nucl Med 2007; 48: pp. 932-945.
144. Cheng G., Torigian D.A., Zhuang H., et. al.: When should we recommend use of dual time-point and delayed time-point imaging techniques in FDG PET?. Eur J Nucl Med Mol Imaging 2013; 40: pp. 779-787.
145. Schillaci O.: Use of dual-point fluorodeoxyglucose imaging to enhance sensitivity and specificity. Semin Nucl Med 2012; 42: pp. 267-280.
146. Hamberg L.M., Hunter G.J., Alpert N.M., et. al.: The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification?. J Nucl Med 1994; 35: pp. 1308-1312.
147. Lodge M., Lucas J., Marsden P., et. al.: A PET study of (18)FDG uptake in soft tissue masses. Eur J Nucl Med 1999; 26: pp. 22-30.
148. Zhuang H., Pourdehnad M., Lambright E.S., et. al.: Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med 2001; 42: pp. 1412-1417.
149. Kumar R., Loving V.A., Chauhan A., et. al.: Potential of dual-time-point imaging to improve breast cancer diagnosis with 18F-FDG PET. J Nucl Med 2005; 46: pp. 1819-1824.
150. Matthies A., Hickeson M., Cuchiara A., et. al.: Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. J Nucl Med 2002; 43: pp. 871-875.
151. Hustinx R., Smith R.J., Benard F., et. al.: Dual time point fluorine-18 fluorodeoxyglucose positron emission tomography: a potential method to differentiate malignancy from inflammation and normal tissue in the head and neck. Eur J Nucl Med 1999; 26: pp. 1345-1348.
152. Lee J.W., Kim S.K., Lee S.M., et. al.: Detection of hepatic metastases using dual-time-point FDG PET/CT scans in patients with colorectal cancer. Mol Imaging Biol 2011; 13: pp. 565-572.
153. Costantini D.L., Vali R., Chan J., et. al.: Dual-time-point FDG PET/CT for the evaluation of pediatric tumors. AJR Am J Roentgenol 2013; 200: pp. 408-413.
154. Berkowitz A., Basu S., Srinivas S., et. al.: Determination of whole-body metabolic burden as a quantitative measure of disease activity in lymphoma: a novel approach with fluorodeoxyglucose-PET. Nucl Med Commun 2008; 29: pp. 521-526.
155. Van de Wiele C., Kruse V., Smeets P., et. al.: Predictive and prognostic value of metabolic tumour volume and total glycolysis in solid tumours. Eur J Nucl Med Mol Imaging 2013; 40: pp. 290-301.
156. Torigian D.A., Zaidi H., Kwee T.C., et. al.: PET/MR imaging: technical aspects and potential clinical applications. Radiology 2013; 267: pp. 26-44.
157. Mavi A., Urhan M., Yu J.Q., et. al.: Dual time point 18F-FDG PET imaging detects breast cancer with high sensitivity and correlates well with histologic subtypes. J Nucl Med 2006; 47: pp. 1440-1446.
![Figure 1, ( Top ) The first tomographic 2-deoxy-2-[ 18 F]fluoro-D-glucose ( 18 F-FDG) images of the brain ( left ) acquired in 1976 at the University of Pennsylvania with a Mark IV scanner designed and built to examine central nervous system disorders with single-γ-emitting radiopharmaceuticals. The image on the right shows a corresponding slice of the brain and its comparable structures. (Reproduced with permission from Seminars in Nuclear Medicine , 2002; 32:2–5.) ( Bottom ) The first whole-body 18 F-FDG images acquired with a dual-head Ohio Nuclear rectilinear scanner equipped with a set of high-energy collimators for performing strontium 85 (with an energy of 510 KeV) bone scans. The image revealed a significant concentration of 18 F-FDG in the brain, heart, and bladder.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ThePivotalRoleofFDGPETCTinModernMedicine/0_1s20S1076633213005163.jpg)
![Figure 2, A 71-year-old female with suspected gastric cancer recurrence. Computed tomography (CT) imaging and endoscopic ultrasound (EUS) showed enlarged retroperitoneal lymph nodes but were inconclusive with regards to malignancy; fine needle aspiration from one such node during EUS was without malignant cells. Subsequent 2-deoxy-2-[ 18 F]fluoro-D-glucose (FDG)–positron emission tomography (PET)/CT was performed and transaxial CT ( top ; arrow ), PET ( middle ; arrow ), and fused PET/CT ( bottom ; arrow ) showed focally increased FDG uptake in an enlarged retroperitoneal lymph node with subsequent histologically confirmed recurrence.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ThePivotalRoleofFDGPETCTinModernMedicine/1_1s20S1076633213005163.jpg)
![Figure 3, A 74-year-old female with lymphoma at baseline after finishing chemotherapy (a) and 4 months later as part of routine control (b) . Multiple 2-deoxy-2-[ 18 F]fluoro-D-glucose (FDG)–avid lesions are observed on the latter, consistent with disseminated disease ( b , left panel ). Note the lack of morphological changes in several lesions on axial computed tomography ( a and b , upper right panels ; arrows ) compared to the intense FDG avidity on fused images ( a and b , lower right panels ; arrows ).](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ThePivotalRoleofFDGPETCTinModernMedicine/2_1s20S1076633213005163.jpg)
![Figure 6, Coronal computed tomography (CT) ( left ), positron emission tomography (PET) ( middle ), and fused PET/CT ( right ) images of a female smoker showing the ascending aorta and common carotid arteries at 2 hours after injection of 2-deoxy-2-[ 18 F]fluoro-D-glucose (FDG). Intense focal FDG accumulation is seen in the ascending aorta ( interrupted arrow ) and right common carotid artery ( arrow ).](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ThePivotalRoleofFDGPETCTinModernMedicine/5_1s20S1076633213005163.jpg)
![Figure 7, A 71-year-old female with nonspecific symptoms and intermittent fever. Maximum intensity projection positron emission tomography (PET) ( left ) and fused sagittal ( upper right ) and transverse ( lower right ) PET/computed tomography images show marked 2-deoxy-2-[ 18 F]fluoro-D-glucose uptake in the large vessels ( black solid arrows ) and the aorta ( white interrupted arrows ), consistent with active large vessel vasculitis and aortitis.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ThePivotalRoleofFDGPETCTinModernMedicine/6_1s20S1076633213005163.jpg)
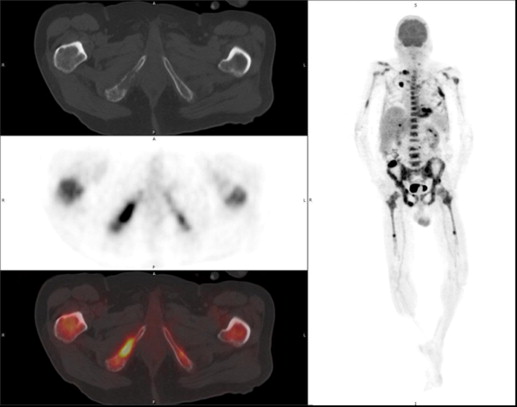
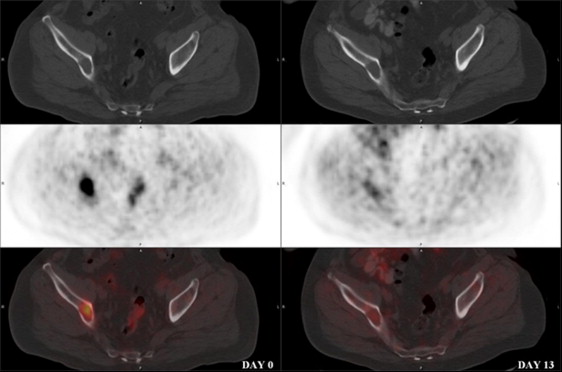
![Figure 8, A 58-year-old male with L3/L4 spondylodiscitis. Fused transaxial/sagittal 2-deoxy-2-[ 18 F]fluoro-D-glucose (FDG) positron emission tomography/computed tomography images show marked FDG uptake (maximum standardized uptake value [SUV MAX ] of 28.7) in the infected disc area at baseline ( left ; arrow ) and remission (SUV MAX of 10.1) after 8 weeks of antibiotics ( right ; interrupted arrow ).](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ThePivotalRoleofFDGPETCTinModernMedicine/7_1s20S1076633213005163.jpg)
![Figure 9, A 37-year-old male with sarcoidosis and intermittent fever. 2-Deoxy-2-[ 18 F]fluoro-D-glucose ( 18 FDG)–positron emission tomography/computed tomography (PET/CT) imaging demonstrated persistent 18 FDG avidity in hilar ( arrows ), periclavicular, and retroperitoneal lymph nodes on the maximum intensity projection ( left ) and transaxial PET/CT ( right ) images.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ThePivotalRoleofFDGPETCTinModernMedicine/8_1s20S1076633213005163.jpg)
![Figure 10, 2-Deoxy-2-[ 18 F]fluoro-D-glucose ( 18 FDG)–positron emission tomography/computed tomography imaging detected multiple 18 F-FDG–avid lesions in liver, spleen, and lung in a patient with metastatic breast cancer ( left ). Semiautomated segmentation allowed calculation of the global disease burden ( right ).](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ThePivotalRoleofFDGPETCTinModernMedicine/9_1s20S1076633213005163.jpg)
![Figure 11, Positron emission tomography/magnetic resonance imaging (PET/MR) demonstrates high sensitivity for detection of small tumor deposits. 2-Deoxy-2-[ 18 F]fluoro-D-glucose (FDG)–PET/MR with diffusion imaging demonstrates increased tracer uptake within a tiny intramuscular melanoma metastasis, also well-delineated on diffusion imaging ( arrow ). ADC, apparent diffusion coefficient map; HASTE, half-Fourier acquisition single-shot turbo spin-echo; STIR, short tau inversion recovery.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ThePivotalRoleofFDGPETCTinModernMedicine/10_1s20S1076633213005163.jpg)
![Figure 12, 2-Deoxy-2-[ 18 F]fluoro-D-glucose (FDG)–positron emission tomography/magnetic resonance (PET/MR) imaging of a patient with metastatic malignant melanoma. FDG-avid left inguinal lymph nodes are precisely delineated on multiple high-contrast (radial volumetric interpolated breath hold examination [VIBE], half-Fourier acquisition single-shot turbo spin-echo [HASTE]) and quantitative (apparent diffusion coefficient map [ADC], diffusion-weighted imaging [DWI]) MR data sets. PET/MR imaging offers increased tissue contrast in the pelvis and allows for better delineation of tumor margins. Simultaneous imaging reduces bladder and bowel motion artifact.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ThePivotalRoleofFDGPETCTinModernMedicine/11_1s20S1076633213005163.jpg)