Rationale and Objectives
To evaluate the feasibility of using diffusion-weighted imaging (DWI) with an array spatial sensitivity encoding technique (ASSET) and apparent diffusion coefficient (ADC) map values with different b values to distinguish benign and malignant breast lesions.
Materials and Methods
Fifty-six female patients with 60 histologically proven breast lesions and 20 healthy volunteers underwent magnetic resonance imaging. A subset of normal volunteers ( n = 7) and patients ( n = 16) underwent both conventional DWI and ASSET-DWI, and the image quality between the two methods was compared. Finally, ASSET-DWI with b = 0, 600 s/mm 2 , and b = 0, 1000 s/mm 2 , were compared for their ability to distinguish benign and malignant breast lesions.
Results
The ASSET-DWI method had less distortion, fewer artifacts, and a lower acquisition time than other methods. No significant difference ( P > .05) was detected in ADC map values between ASSET-DWI and conventional DWI. For ASSET-DWI, the sensitivity of ADC values for malignant lesions with a threshold of less than 1.44 × 10 −3 mm 2 /s (b = 600 s/mm 2 ) and 1.18 × 10 −3 mm 2 /s (b = 1000 s/mm 2 ) was 80% and 77.5%, respectively. The specificity of both groups was 95%.
Conclusion
ASSET-DWI evaluation of breast tissue offers decreased distortion, susceptibility to artifacts, and acquisition time relative to other methods. The use of ASSET-DWI is feasible with b values ranging from 600 to 1000 s/mm 2 and provides increased specificity compared to other techniques. Thus, the ADC value of a breast lesion can be used to further characterize malignant lesions from benign ones.
Magnetic resonance imaging (MRI) is becoming an essential tool for examination of breast cancer tissue; compared to ultrasound and mammography, it has remarkably high sensitivity due to the use of contrast enhancement material . Recent multicenter trials have established that dynamic gadolinium contrast enhanced magnetic resonance imaging (DCE-MR) has high sensitivity (>90%) and moderate specificity (∼85%) . Some benign lesions exhibit contrast characteristics that are similar to those of malignant lesions (eg, fibroadenomas) . Therefore, increasing the specificity is a challenge for breast MRI and other imaging methods.
Diffusion-weighted imaging (DWI) is a specific type of MRI. Diffusion is a physical phenomenon that differs from conventional parameters, such as T1 and T2. The principle that underlies DWI is that the thermal motion of the water molecules in the extracellular fluid enables the acquisition of an image that reflects both histological structure and cellularity. DWI is sensitive to changes in the micro-diffusion of water within the intra- and intercellular environments . After an event that has caused a disruption or restriction of the flow of water within a tissue, such as ischemic events (eg, stroke) or tumor growth, cytotoxic edema occurs and results in changes in the diffusion of water within the tissue . These changes in the diffusion of water result in changes in the signal intensity on the DWI (either hyperintensity or hypointensity) . DWI also provides a quantitative biophysical parameter called the apparent diffusion coefficient of water (ADC) map. The ADC map is an indicator of the movement of water within the tissue. It gives an average value of the flow and the distance that a water molecule has moved, and it has been related to the state of tissue during the evolution of cerebral ischemia and tumor progression .
Get Radiology Tree app to read full this article<
Materials and methods
Get Radiology Tree app to read full this article<
Clinical Subjects
Get Radiology Tree app to read full this article<
Clinical and Histological Analysis
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
MRI Protocol
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
MRI Data Analysis
Get Radiology Tree app to read full this article<
Measurement of ADC Values
Get Radiology Tree app to read full this article<
Measurement of the Signal-to-noise Ratio of the Normal Breast Tissue and the Contrast-to-noise Ratio of the Breast Lesions
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
SNRn=Sn/SDb SNR
n
=
S
n
/
SD
b
where SNR n is the SNR of the normal breast tissue, S n is the signal intensity of normal breast tissue, and SD b is the standard deviation of the noise in the background.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
CNR1=(S1−Sn)/SDb CNR
1
=
(
S
1
-
S
n
)
/
SD
b
where CNR l = CNR of the lesion in the breast, S l is the signal intensity of the lesion, S n is the signal intensity of normal breast tissue, and SD b is the standard deviation of the noise in the background.
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
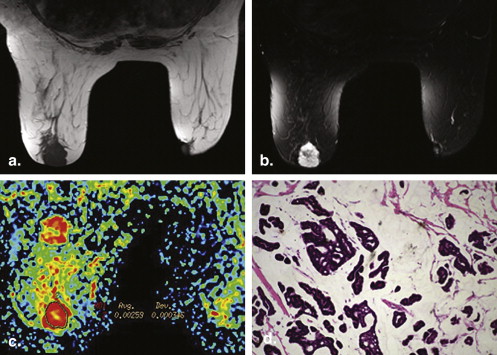
Comparison of ASSET-DWI and Conventional DWI
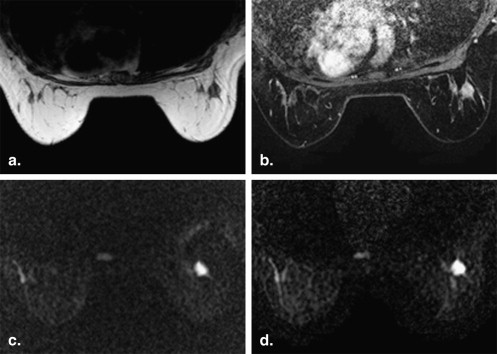
Image artifacts
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
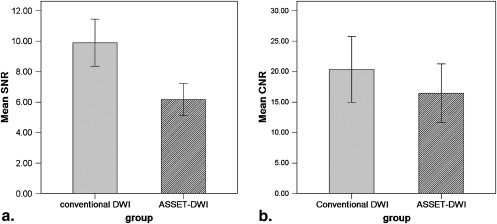
SNR and CNR
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Mean ADC value
Get Radiology Tree app to read full this article<
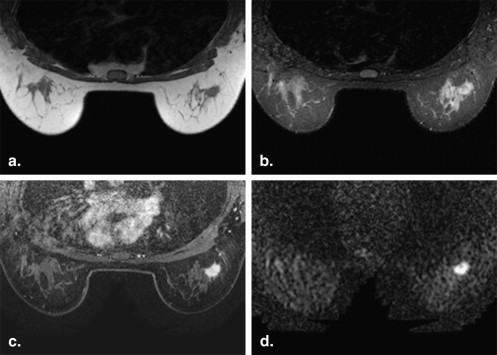
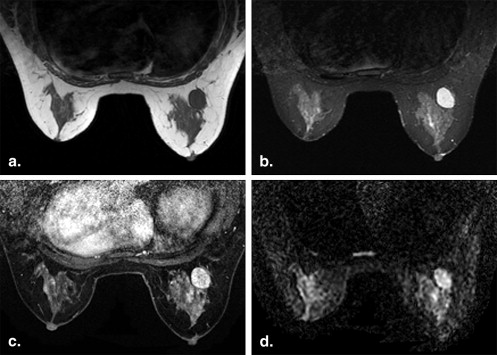
Lesion Detection by ASSET-DWI with Different b Values
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Table 1
The Mean Apparent Diffusion Coefficient Values of Benign Lesions, Malignant Lesions, and Normal Breast Tissue Obtained Using Different b Values
Group_n_ Mean Apparent Diffusion Coefficient Value (×10 −3 mm 2 /s) Range of 95% Confidence (×10 −3 mm 2 /s) b = 600 s/mm 2 Malignant 40 1.33 ± 0.36 ∗ , † 1.21–1.44 Benign 20 1.82 ± 0.31 ‡ 1.68–1.97 Normal 20 2.05 ± 0.33 1.90–2.21 b = 1000s/mm 2 Malignant 40 1.08 ± 0.32 ∗ , † 0.97–1.18 Benign 20 1.61 ± 0.33 ‡ 1.45–1.76 Normal 20 1.85 ± 0.33 1.70–2.0
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
The Study Demonstrated that using ASSET-DWI Decreased Known EPI Distortions and Provided Excellent Differentiation of Malignant from Benign Lesions
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
The b Value and the Diagnostic Threshold of the ADC Value in ASSET-DWI
Get Radiology Tree app to read full this article<
Limitations of the Study
Get Radiology Tree app to read full this article<
Conclusions
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Heywang S.H., Fenzl G., Beck R., et. al.: Use of Gd-DTPA in the nuclear magnetic resonance study of the breast. Rofo 1986; 145: pp. 565-571.
2. Kaiser W.A., Zeitler E.: MR imaging of the breast: fast imaging sequences with and without Gd-DTPA. Preliminary observations. Radiology 1989; 170: pp. 681-686.
3. Kuhl C.K., Mielcareck P., Klaschik S., et. al.: Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions?. Radiology 1999; 211: pp. 101-110.
4. Lehman C.D., Schnall M.D.: Imaging in breast cancer: magnetic resonance imaging. Breast Cancer Res 2005; 7: pp. 215-219.
5. Bluemke D.A., Gatsonis C.A., Chen M.H., et. al.: Magnetic resonance imaging of the breast prior to biopsy. JAMA 2004; 292: pp. 2735-2742.
6. Choi N., Han B.K., Choe Y.H., et. al.: Three-phase dynamic breast magnetic resonance imaging with two-way subtraction. J Comput Assist Tomogr 2005; 29: pp. 834-841.
7. Le Bihan D., Breton E., Lallemand D., et. al.: MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986; 161: pp. 401-407.
8. Barber P.A., Hill M.D., Eliasziw M., et. al.: Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Psychiatry 2005; 76: pp. 1528-1533.
9. Chenevert T.L., Stegman L.D., Taylor J.M., et. al.: Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst 2000; 92: pp. 2029-2036.
10. Kucharczyk J., Mintorovitch J., Asgari H.S., et. al.: Diffusion/perfusion MR imaging of acute cerebral ischemia. Magn Reson Med 1991; 19: pp. 311-315.
11. Brunberg J.A., Chenevert T.L., McKeever P.E., et. al.: In vivo MR determination of water diffusion coefficients and diffusion anisotropy: correlation with structural alteration in gliomas of the cerebral hemispheres. AJNR Am J Neuroradiol 1995; 16: pp. 361-371.
12. Englander S.A., Uluğ A.M., Brem R., et. al.: Diffusion imaging of human breast. NMR Biomed 1997; 10: pp. 348-352.
13. Guo Y., Cai Y.Q., Cai Z.L., et. al.: Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reason Imaging 2002; 16: pp. 172-178.
14. Sinha S., Lucas-Quesada F.A., Sinha U., et. al.: In vivo diffusion-weighted MRI of the breast: potential for lesion characterization. J Magn Reson Imaging 2002; 15: pp. 693-704.
15. Kinoshita T., Yashiro N., Ihara N., et. al.: Diffusion-weighted half-Fourier single-shot turbo spin echo imaging in breast tumor: differentiation of invasive ductal carcinoma from fibroadenoma. J Comput Assist Tomogr 2002; 26: pp. 1042-1046.
16. Woodhams R., Matsunaga K., Iwabuchi K., et. al.: Diffusion-weighted imaging of malignant breast tumors: the usefulness of apparent diffusion coefficient (ADC) value and ADC map for the detection of malignant breast tumors and evaluation of cancer extension. J Comput Assist Tomogr 2005; 29: pp. 644-649.
17. Park M.J., Cha E.S., Kang B.J., et. al.: The role of diffusion-weighted imaging and the apparent diffusion coefficient (ADC) values for breast tumors. Korean J Radiol 2007; 8: pp. 390-396.
18. Kuroki Y., Nas K., Kuroki S., et. al.: Diffusion-weighted imaging of breast cancer with the sensitivity encoding technique: analysis of the apparent diffusion coefficient value. Magn Reson Med Sci 2004; 3: pp. 79-85.
19. Humphries P.D., Sebire N.J., Siegel M.J., et. al.: Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology 2007; 245: pp. 848-854.
20. Squillaci E., Manenti G., Cova M., et. al.: Correlation of diffusion-weighted MR imaging with cellularity of renal tumours. Anticancer Res 2004; 24: pp. 4175-4179.
21. Manenti G., Di Roma M., Mancino S., et. al.: Malignant renal neoplasms: correlation between ADC values and cellularity in diffusion weighted magnetic resonance imaging at 3 T. Radiol Med 2008; 113: pp. 199-213.
22. Sugahara T., Korogi Y., Kochi M., et. al.: Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 1999; 9: pp. 53-60.
23. Hatakenaka M., Soeda H., Yabuuchi H., et. al.: Apparent diffusion coefficients of breast tumors: clinical application. Magn Reson Med Sci 2008; 7: pp. 23-29.
24. Ducatman B.S., Emery S.T., Wang H.H.: Correlation of histologic grade of breast carcinoma with cytologic features on fine-needle aspiration of the breast. Mod Pathol 1993; 6: pp. 539-543.
25. Woodhams R., Matsunaga K., Kan S., et. al.: ADC mapping of benign and malignant breast tumors. Magn Reson Med Sci 2005; 4: pp. 35-42.
26. Le Bihan D., Breton E., Lallemand D., et. al.: Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988; 168: pp. 497-505.
27. Morvan D.: In vivo measurement of diffusion and pseudo-diffusion in skeletal muscle at rest and after exercise. Magn Reson Imaging 1995; 13: pp. 193-199.
28. Thoeny H.C., De Keyzer F., Boesch C., et. al.: Diffusion-weighted imaging of the parotid gland: Influence of the choice of b-values on the apparent diffusion coefficient value. J Magn Reson Imaging 2004; 20: pp. 786-790.
29. Wheeler-Kingshott C.A., Thomas D.L., Lythgoe M.F., et. al.: Burst excitation for quantitative diffusion imaging with multiple b-values. Magn Reson Med 2000; 44: pp. 737-745.