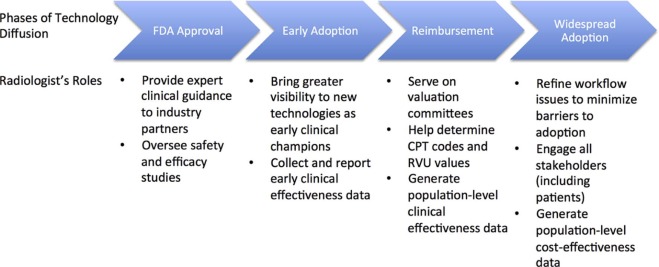
Radiology continues to benefit from constant innovation and technological advances. However, for promising new imaging technologies to reach widespread clinical practice, several milestones must be met. These include regulatory approval, early clinical evaluation, payer reimbursement, and broader marketplace adoption. Successful implementation of new imaging tests into clinical practice requires active stakeholder engagement and a focus on demonstrating clinical value during each phase of translation.
Introduction
Medical imaging plays a central role in screening, diagnosis, and treatment management. Imaging technology has advanced at a tremendous pace, and new imaging techniques are being employed in an increasing number of clinical scenarios . Physicians rely on the power of medical imaging to guide diagnoses and treatment plans. Surgeons and interventional radiologists are increasingly using novel imaging techniques to guide percutaneous and intraoperative procedures. However, even after years of corporate development, many novel imaging applications face multiple additional hurdles before widespread clinical adoption. These include regulatory approval, robust clinical evaluation, and third-party reimbursement.
As technological innovation is the cornerstone of medical imaging, radiology investigators should possess a basic understanding of how novel imaging technologies are translated into clinical practice. Individual radiologists can play a critical role in each step of technology development and clinical adoption. To provide an overview of this important topic to the general radiology audience, the Radiology Research Alliance Task Force on Translating New Imaging Technologies into Clinical Practice was convened to produce this white paper. The objectives of this effort were to provide a synopsis of the technology adoption pathway after manufacturer development, and to encourage radiologists to more effectively engage in bringing new advanced imaging techniques to clinical use, further advancing the field of imaging.
This white paper discusses the following four major phases that new imaging technologies must pass through before incorporation into routine clinical practice: (1) federal regulatory approval, (2) early adoption, (3) payment coverage, and (4) broad adoption ( Fig 1 ). For each of these phases, we introduce standard terminology and major milestones, and stress the important roles that radiologists can play in facilitating the translation of new imaging technologies from bench to bedside. We use digital breast tomosynthesis (DBT), a recently adopted advanced imaging technique, as an example across these different phases. Finally, we conclude with a brief discussion of policy-related and political barriers to imaging technology adoption, highlighting the need for active radiologist engagement at the earliest phases of translational research.
Food and Drug Administration (FDA) Approval Phase
Imaging technologies are considered medical devices under the Federal Food, Drug, and Cosmetic Act of 1938 and as amended by the Medical Device Regulation Act of 1976. Under federal regulation, the U.S. FDA, through its Center for Devices and Radiological Health, is tasked with overseeing the domestic production, the distribution, and sales of medical imaging devices. FDA approval is necessary before a manufacturer can distribute and market a new imaging device. Medicare reimbursement also requires FDA approval. Even though the FDA permits off-label use of a device at an individual physician’s discretion, a manufacturer cannot market off-label indications. Additionally, federal reimbursement is often more challenging without approval for specific clinical indications .
Medical Device Classes
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Safety and Effectiveness
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Early Adoption Phase
Get Radiology Tree app to read full this article<
Radiology-Industry Partnerships
Get Radiology Tree app to read full this article<
Trialability and Compatibility
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Reimbursement Phase
Get Radiology Tree app to read full this article<
Code Development
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Service Valuation
Get Radiology Tree app to read full this article<
Medicare Coverage and Reimbursement
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Broad Adoption Phase
Get Radiology Tree app to read full this article<
Infrastructure and Workflow
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Stakeholder Engagement and Advocacy Efforts
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Demonstrating Value
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Conclusion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Reiner B.I.: Optimizing technology development and adoption in medical imaging using the principles of innovation diffusion, part II: practical applications. J Digit Imaging 2012; 25: pp. 7-10.
2. Kopans D.B.: “Off label” use of FDA-approved devices and digital breast tomosynthesis. AJR Am J Roentgenol 2015; 205: pp. 1149-1151.
3. U.S. Food and Drug Administration : Overview of device regulation. Available at http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/
4. Smith J.J.: Regulation of medical devices in radiology: current standards and future opportunities. Radiology 2001; 218: pp. 329-335.
5. Gunderman R.B., Meesa I.R.: The adoption of innovation. Radiology 2008; 246: pp. 659-661.
6. Rogers E.M.: Diffusion of innovations.5th ed.2003.Free PressNew York, NY
7. Rafferty E.A., Park J.M., Philpotts L.E., et. al.: Assessing radiologist performance using combined digital mammography and breast tomosynthesis compared with digital mammography alone: results of a multicenter, multireader trial. Radiology 2013; 266: pp. 104-113.
8. Greenberg J.S., Javitt M.C., Katzen J., et. al.: Clinical performance metrics of 3D digital breast tomosynthesis compared with 2D digital mammography for breast cancer screening in community practice. AJR Am J Roentgenol 2014; 203: pp. 687-693.
9. Lee C.I., Lehman C.D.: Digital breast tomosynthesis and the challenges of implementing an emerging breast cancer screening technology into clinical practice. J Am Coll Radiol 2013; 10: pp. 913-917.
10. Leslie-Mazwi T.M., Bello J.A., Tu R., et. al.: Current procedural terminology: history, structure, and relationship to valuation for the neuroradiologist. AJNR Am J Neuroradiol 2016;
11. Thorwarth W.T.: CPT: an open system that describes all that you do. J Am Coll Radiol 2008; 5: pp. 555-560.
12. Thorwarth W.T.: From concept to CPT code to compensation: how the payment system works. J Am Coll Radiol 2004; 1: pp. 48-53.
13. Hirsch J.A., Leslie-Mazwi T.M., Nicola G.N., et. al.: Current procedural terminology; a primer. J Neurointerv Surg 2015; 7: pp. 309-312.
14. Cox K., Duszak R., Hemingway J., et. al.: Reassessing Medicare trends in diagnostic CT colonography after achieving CPT code category I status. Abdom Radiol (NY) 2016; 41: pp. 1357-1362.
15. Duszak R., Optican R.J., Brin K.P., et. al.: Cardiac CT and coronary CTA: early Medicare claims analysis of national and regional utilization and coverage. J Am Coll Radiol 2011; 8: pp. 549-555.
16. Duszak R., Kim D.H., Pickhardt P.J.: Expanding utilization and regional coverage of diagnostic CT colonography: early Medicare claims experience. J Am Coll Radiol 2011; 8: pp. 235-241.
17. Hsiao W.C., Braun P., Dunn D.L., et. al.: An overview of the development and refinement of the Resource-Based Relative Value Scale. The foundation for reform of U.S. physician payment. Med Care 1992; 30: pp. NS1-12.
18. Hsiao W.C., Braun P., Dunn D., et. al.: Resource-based relative values. An overview. JAMA 1988; 260: pp. 2347-2353.
19. UCSF Medical Group: RVU & RBRVS basics. Available at http://medgroup.ucsf.edu/data-warehouse/rvu-rbrvs-basics
20. American Medical Association : RBRVS overview. Available at https://www.ama-assn.org/rbrvs-overview
21. Otero H.J., Chambers J.D., Bresnahan B.W., et. al.: Medicare’s national coverage determinations in diagnostic radiology: examining evidence and setting limits. Acad Radiol 2012; 19: pp. 1060-1065.
22. Social Security Administration : Compilation of the social security laws. Available at http://www.ssa.gov/OP_Home/ssact/title18/1862.htm
23. Centers for Medicare and Medicaid Services : Revised process for making Medicare national coverage determinations.2003.
24. Centers for Medicare and Medicaid Services : Medicare coverage policy—MCAC charter. Available at http://www.cms.gov/mcac/default.asp
25. Centers for Medicare and Medicaid Services : Guidance for the public, industry and CMS staff. Factors CMS considers in opening a national coverage determination 2006. Available at https://www.cms.gov/medicarecoverage-database/details/medicare-coverage-document-details.aspx
26. National Lung Screening Trial Research Team, Aberle D.R., Adams A.M., et. al.: Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365: pp. 395-409.
27. Hardesty L.A.: Issues to consider before implementing digital breast tomosynthesis into a breast imaging practice. AJR Am J Roentgenol 2015; 204: pp. 681-684.
28. Houssami N., Skaane P.: Overview of the evidence on digital breast tomosynthesis in breast cancer detection. Breast 2013; 22: pp. 101-108.
29. Lee C.I., Bassett L.W., Lehman C.D.: Breast density legislation and opportunities for patient-centered outcomes research. Radiology 2012; 264: pp. 632-636.
30. Lee C.I., Jarvik J.G.: Patient-centered outcomes research in radiology: trends in funding and methodology. Acad Radiol 2014; 21: pp. 1156-1161.
31. Skaane P., Bandos A.I., Gullien R., et. al.: Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267: pp. 47-56.
32. Ciatto S., Houssami N., Bernardi D., et. al.: Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14: pp. 583-589.
33. Friedewald S.M., Rafferty E.A., Rose S.L., et. al.: Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311: pp. 2499-2507.
34. Oeffinger K.C., Fontham E.T., Etzioni R., et. al.: Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015; 314: pp. 1599-1614.
35. Siu A.L., Force USPST: Screening for breast cancer: U.S. preventive services task force recommendation statement. Ann Intern Med 2016; 164: pp. 279-296.
36. Pandharipande P.V., Gazelle G.S.: Comparative effectiveness research: what it means for radiology. Radiology 2009; 253: pp. 600-605.
37. Lee C.I., Cevik M., Alagoz O., et. al.: Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology 2015; 274: pp. 772-780.
38. American College of Radiology : ACR advocates for breast tomosynthesis coverage. Available at https://www.acr.org/Advocacy/eNews/20160812-Issue/20160812-ACR-Advocates-for-Breast-Tomosynthesis-Coverage