Rationale and Objectives
The aim of this study was to explore the antitumor effects on mice xenografted ovarian carcinoma using the technique of ultrasound-mediated drug release from paclitaxel-loaded lipid microbubbles (PLMs).
Materials and Methods
Twenty-five ovarian cancer–bearing nude mice were randomly divided into five groups of five mice each. Each group received a unique kind of treatment once a day. These treatments were PLMs combined with ultrasound, intravenous paclitaxel administration, non–drug-loaded microbubbles combined with ultrasound, intravenous PLM administration, and normal saline administration (the control group). After 7 days of consecutive treatment, all mice were sacrificed, and their tumors were harvested to measure volumes and weights. The tumor inhibition rate was calculated by weight. Expressions of vascular endothelial growth factor (VEGF) and p53 in tumor tissues were detected by immunohistochemical staining.
Results
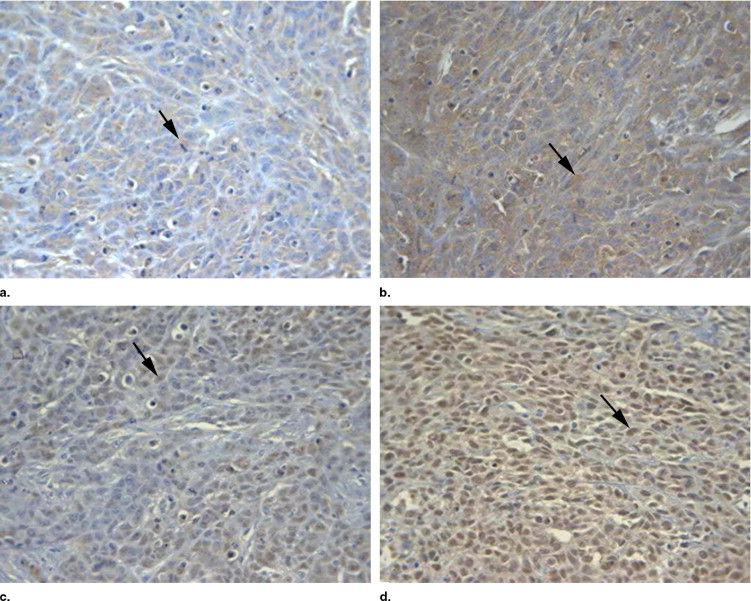
Mean tumor volume and weight were the lowest in the first group (PLMs combined with ultrasound), so this group’s tumor inhibition rate was the highest ( P < .05). On immunohistology, VEGF and p53 expression levels were lowest ( P < .05) in the first group.
Conclusion
Ultrasound irradiation mediates PLM destruction so that the drug is released from the vehicles at the same time. It helps achieve targeted chemotherapy in tumor tissues. This technique has potential to be adopted as a novel tool for ovarian cancer chemotherapy.
Ovarian carcinoma is the leading cause of death in patients with gynecologic malignancies ( ). Because of the insidious onset of the disease and the lack of reliable screening tests, the majority of patients already have advanced disease when diagnosed. Although most patients respond initially to the standard treatment of modern surgical intervention and platinum or paclitaxel chemotherapy, because intravenous (IV) chemotherapy intensity is limited by myelotoxicity and other serious side effects, the majority of patients will eventually relapse and die of the disease ( ).
Another approach to the treatment of women with advanced ovarian cancer is the direct instillation of chemotherapy into the peritoneal cavity. First proposed by Dedrick et al ( ), intraperitoneal (IP) therapy is designed to maximize drug delivery to the tumor while avoiding much of the systemic toxicity associated with the IV administration of the drug. In particular, the combined application of IV and IP chemotherapy can greatly improve the median progression-free survival time ( ). However, the toxicity resulting from this method of therapy is not trivial. Apart from the most common toxicities, such as neutropenia, nausea, vomiting, and fatigue, researchers have also observed abdominal pain, catheter-related infections, and so on ( ). Also, IP therapy calls for such high familiarity with peritoneal administration and catheter-placement techniques that many clinicians are reluctant to embrace it. Therefore, a novel form of chemotherapy with improved outcomes is needed for the treatment of ovarian cancer.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Materials and methods
Preparation of Paclitaxel-loaded Microbubbles
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Animal Preparation
Get Radiology Tree app to read full this article<
Treatment
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Tumor Growth Measurement
Get Radiology Tree app to read full this article<
Immunohistochemical Staining of VEGF and p53
Get Radiology Tree app to read full this article<
Image Analysis
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Animal Models
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Effects of Treatments on Tumor Growth
Get Radiology Tree app to read full this article<
Table 1
Tumor Growth Suppression in Each Group
Group_n_ Tumor Volume (mm 3 ) Tumor Weight (mg) Tumor Inhibition Rate (%) 1 5 918.1 ± 249.2 ⁎ , † 825.6 ± 214.6 ⁎ , † 45.2 2 5 1,128.1 ± 178.4 ⁎ 956.1 ± 138.6 ⁎ 36.5 3 5 1,435.4 ± 249.7 ⁎ 1,234.9 ± 139.0 ⁎ 18.0 4 5 1,724.9 ± 278.2 ⁎ 1,478.7 ± 242.4 ⁎ 5.9 5 5 1,752.9 ± 332.2 1,506.7 ± 304.4 0
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Expression of VEGF and p53 in Tumor Tissues
Get Radiology Tree app to read full this article<
Table 2
Vascular Endothelial Growth Factor (VEGF) and p53 Expression in Each Group
Group_n_ VEGF Slide Density p53 Slide Density 1 5 0.13524 ± 0.00162 ⁎ , † 0.12228 ± 0.00193 ⁎ , † 2 5 0.17455 ± 0.00231 ⁎ 0.18333 ± 0.00480 ⁎ 3 5 0.19246 ± 0.00583 ⁎ 0.20653 ± 0.00329 ⁎ 4 5 0.22470 ± 0.00751 ⁎ 0.23587 ± 0.001469 ⁎ 5 5 0.26938 ± 0.00672 0.33093 ± 0.00550
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Jemal A., Siegel R., Ward E., et. al.: Cancer statistics, 2006. CA Cancer J Clin 2006; 56: pp. 106-130.
2. Ozols R.F.: Challenges for chemotherapy in ovarian cancer. Ann Oncol 2006; 17: pp. 181-187.
3. Dedrick R.L., Myers C.E., Bungay P.M., DeVita V.T.: Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep 1978; 62: pp. 1-11.
4. Dedrick R.L., Flessner M.F.: Pharmacokinetic problems in peritoneal drug administration: tissue penetration and surface exposure. J Natl Cancer Inst 1997; 89: pp. 480-487.
5. Markman M., Bundy B.N., Alberts D.S., et. al.: Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 2001; 19: pp. 1001-1007.
6. Rothenberg M.L., Liu P.Y., Braly P.S., et. al.: Combined intraperitoneal and intravenous chemotherapy for women with optimally debulked ovarian cancer: results from an intergroup phase II trial. J Clin Oncol 2003; 21: pp. 1313-1319.
7. Tiukinhoy S.D., Mahowald M.E., Shively V.P., et. al.: Development of echogenic, plasmid-incorporated, tissue-targeted cationic liposomes that can be used for directed gene delivery. Invest Radiol 2000; 35: pp. 732-738.
8. Li T., Tachibana K., Kuroki M., Kuroki M.: Gene transfer with echo-enhanced contrast agents: comparison between Albunex, Optison, and Levovist in mice—initial results. Radiology 2003; 229: pp. 423-428.
9. Amura M., Sato N., Taniyama Y., et. al.: Development of efficient plasmid DNA transfer into adult rat central nervous system using microbubble-enhanced ultrasound. Gene Ther 2004; 11: pp. 1532-1539.
10. Zhigang W., Zhiyu L., Haitao R., et. al.: Ultrasound-mediated microbubble destruction enhances VEGF gene delivery to the infarcted myocardium in rats. Clin Imaging 2004; 28: pp. 395-398.
11. Zhang Q., Wang Z., Ran H., et. al.: Enhanced gene delivery into skeletal muscles with ultrasound and microbubble techniques. Acad Radiol 2006; 13: pp. 363-367.
12. Yang C., Wang Z., Li X., et. al.: Preparation of acoustically active lipospheres containing paclitaxel. Chinese J Ultrasonogr 2006; 15: pp. 535-538.
13. Yang C., Wang Z., Lai T., Li P., Peng X., Xie Z.: Study on cytotoxic effect and acute toxicity of self-made paclitaxel-carrying lipospheres. Chinese J Ultrasound Med 2006; 22: pp. 481-483.
14. Mitragotri S., Blankschtein D., Langer R.: Transdermal drug delivery using low-frequency sonophoresis. Pharmaceut Res 1996; 13: pp. 411-420.
15. Mitragotri S., Kost J.: Low-frequency sonophoresis: a noninvasive method of drug delivery and diagnostics. Biotechnol Prog 2000; 16: pp. 488-492.
16. Tachibana K., Uchida T., Tamura K., Eguchi H., Yamashita N., Ogawa K.: Enhanced cytotoxic effect of Ara-C by low intensity ultrasound to HL-60 cells. Cancer Lett 2000; 149: pp. 189-194.
17. Munshi N., Rapoport N., Pitt W.G.: Ultrasonic activated drug delivery from Pluronic P-105 micelles. Cancer Lett 1997; 117: pp. 1-7.
18. Yu T.H., Hu K., Bai J., Wang Z.B.: Reversal of adriamycin resistance in ovarian carcinoma cell line by combination of verapamil and low-level ultrasound. Ultrason Sonochemy 2003; 10: pp. 37-40.
19. Marmottant P., Hilgenfeldt S.: Controlled vesicle deformation and lysis by single oscillating bubbles. Nature 2003; 423: pp. 153-156.
20. Hernot S., Klibanov A.L.: Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev 2008; 60: pp. 1153-1166.
21. Park S.I., Shah A.N., Zhang J., et. al.: Regulation of angiogenesis and vascular permeability by Src family kinases: opportunities for therapeutic treatment of solid tumors. Exp Opin Ther Targets 2007; 11: pp. 1207-1217.
22. Lee J.S., Choi Y.D., Lee J.H., et. al.: Expression of cyclooxygenase-2 in epithelial ovarian tumors and its relation to vascular endothelial growth factor and p53 expression. Int J Gynecol Cancer 2006; 16: pp. 247-253.
23. Loo W.T., Fong J.H., Cheung M.N., Chow L.W.: The efficacy of paclitaxel on solid tumour analysed by ATP bioluminescence assay and VEGF expression: a translational research study. Biomed Pharmacother 2005; 59: pp. S337-S339.
24. Niu Q., Tang Z.Y., Ma Z.C., et. al.: Serum vascular endothelial growth factor is a potential biomarker of metastatic recurrence after curative resection of hepatocellular carcinoma. World J Gastroenterol 2000; 6: pp. 565-568.
25. Ueno Y.: Implication of vascular endothelial growth factor (VEGF) in human head and neck cancer. Nippon Jibiinkoka Gakkai Kaiho 2006; 109: pp. 163-170.
26. Inan S., Vatansever S., Celik-Ozenci C., et. al.: Immunolocalizations of VEGF, its receptors flt-1, KDR, and TGF-beta’s in epithelial ovarian tumors. Histol Histopathol 2006; 21: pp. 1055-1064.
27. Kim S.Y., Kim J.Y., Kim S.H., et. al.: Surfactin from Bacillus subtilis displays anti-proliferative effect via apoptosis induction, cell cycle arrest and survival signaling suppression. FEBS Lett 2007; 581: pp. 865-871.
28. Secord A.A., Darcy K.M., Hutson A., et. al.: Co-expression of angiogenic markers and associations with prognosis in advanced epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol 2007; 106: pp. 221-232.