Rationale and Objectives
To prospectively evaluate and compare the accuracy of contrast-enhanced spectral mammography (CESM) and ultrasound (US) in size measurement of breast cancer with histologic tumor sizes as gold standard.
Materials and Methods
Twenty women aged between 40–73 years (mean age, 57 ± 10 years) with histologically proven invasive ductal/lobular carcinomas were included in the study. Agreement between imaging tumor size (CESM and US) and histopathologic tumor size was evaluated with Bland–Altman analysis. Stereotactically guided vacuum biopsy was performed in four patients after CESM. Two independent reviewers described artifacts of CESM.
Results
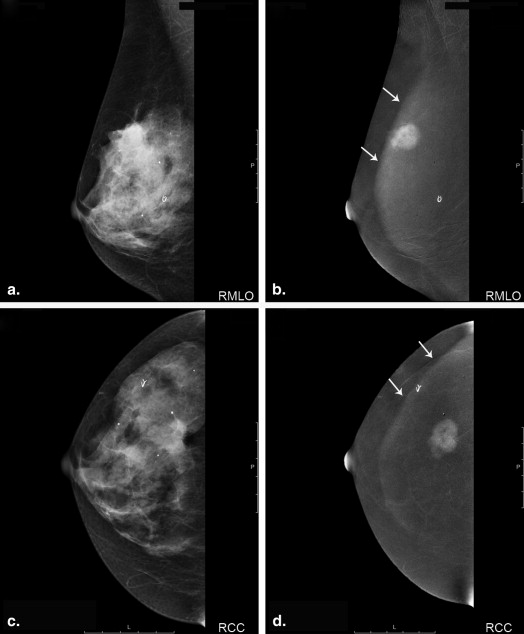
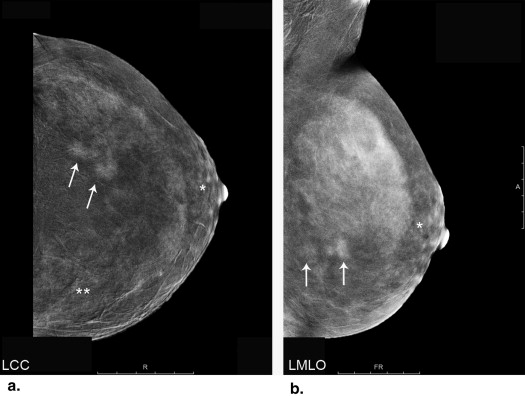
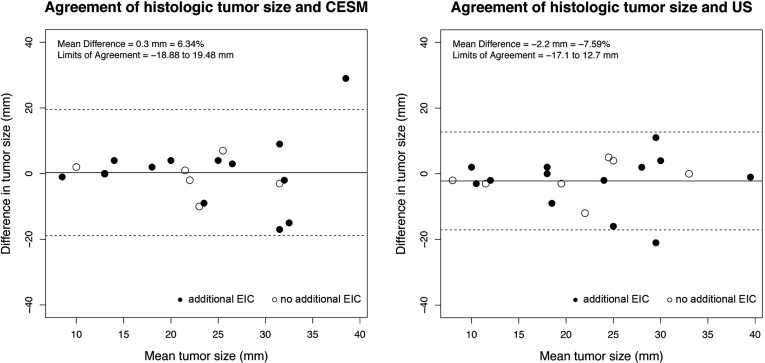
Motion artifacts did not occur in the study. CESM-specific artifacts caused by scattered radiation mostly occurred in oblique view of CESM. Background enhancement of breast tissue was seen in four patients. Mean difference of tumor sizes was 0.3 mm (6.34%) between CESM and histology and −2.2 mm (−7.59%) between US and histology. Limits of agreement ranged from −18.9 to 19.48 mm for CESM and from −17.1 to 12.7 mm with US. Especially smaller tumors with a size <23 mm were measured more precisely with CESM. Enhancement of breast tissue around microcalcifications correlated with abnormalities.
Conclusions
CESM is accurate in size measurements of small breast tumors. On average CESM leads to a slight overestimation of tumor size, whereas US tends to underestimate tumor size. Assessment of the breast tissue can be limited by the scattered radiation artifact and background enhancement of breast tissue. CESM seems to be helpful in the characterization of breast tissue around microcalcifications.
Contrast-enhanced spectral mammography (CESM) is an imaging technique combining digital mammography with intravenous injection of iodinated contrast media to detect hypervascularized lesions, especially in dense breast tissue . Although it was developed several years ago, knowledge about the performance of this technique in clinical routine, especially in breast cancer screening, is still limited. However, a recent study proposes similar indications for CESM as for magnetic resonance imaging (MRI), namely preoperative staging, detection of occult lesions, monitoring of treatment response . Initial results comparing this new technique with mammography, ultrasound (US) and breast MRI show a better detection of suspicious lesions with CESM compared to full-field digital mammography and the combined imaging of mammography and US but a lower detection rate of hypervascularized breast lesions compared to breast MRI . Studies evaluating the accuracy of CESM compared to MRI in preoperative tumor staging show a similar accuracy in lesion size measurement compared to MRI .
Preoperative staging of cancer extent in the breast is necessary to plan the optimal treatment . MRI is the most commonly used approach to determine the extent of the tumor in the breast and to decide which surgery should be performed and if the breast should be radiated . Besides its high cost and limited availability, one major problem of breast MRI is background enhancement of breast tissue, which decreases the detection of breast lesions and affects breast cancer staging . In such patients, US is a good alternative method for breast cancer staging. Studies correlating tumor size determined with imaging and histopathology describe a tendency to underestimate tumor size with US , whereas MRI tends to overestimate tumor size . The good performance of CESM in the detection of multifocal and multicentric cancer dissemination has been shown , as well as the high accuracy of tumor size measurements with CESM compared to MRI . The performance of CESM in preoperative tumor size measurement compared to US has not been investigated so far.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Material and methods
Get Radiology Tree app to read full this article<
Patients
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
CESM Imaging
Get Radiology Tree app to read full this article<
Ultrasound
Get Radiology Tree app to read full this article<
Image Analysis
Contrast-Enhanced Spectral Mammography
Get Radiology Tree app to read full this article<
Statistical Analysis
Get Radiology Tree app to read full this article<
Results
Get Radiology Tree app to read full this article<
Table 1
Details of Patients Included in the Study
Number of Patients Age (Mean Age; Standard Deviation) Disease ACR (Artifacts) ACR2 ACR3 ACR 4 20 40–73 (57; 10) Invasive cancer 2 4 1 Invasive cancer with EIC 4 (1) 6 3 (2)
The majority of patients had invasive cancer with additional extensive in-situ component (EIC).
Get Radiology Tree app to read full this article<
Artifacts
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Background Parenchymal Enhancement of Breast Tissue
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
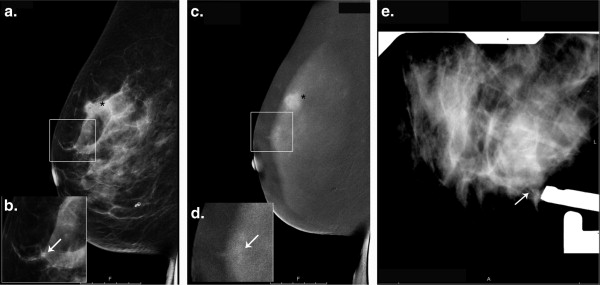
CESM Before Stereotactically Guided Vacuum-Assisted Core Biopsy of the Breast
Get Radiology Tree app to read full this article<
Table 2
Histopathologic Results of Four Patients with Suspicious Microcalcifications, Which Received CESM before Stereotactic-Guided Vacuum Biopsy
Patients Primary Cancer Type Enhancement Around Microcalcifications Histopathologic Finding Invasive lobular cancer N Fibrocystic mastopathy, ductal hyperplasia, and sclerosing adenosis N Fibrocystic mastopathy, ductal hyperplasia, and sclerosing adenosis Invasive ductal cancer N Fibrocystic mastopathy, ductal hyperplasia, and chronic inflammation Invasive ductal cancer Y Atypical lobular hyperplasia (ALH) ∗ N Fibrocystic mastopathy and ductal hyperplasia Invasive ductal cancer Y DCIS ∗
CESM, contrast-enhanced spectral mammography; DCIS, ductal carcinoma in situ.
CESM showed enhancement surrounding microcalcifications in two patients, one with DCIS and one with ALH. Both lesions were excised.
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
No Significant Influence of Additional EIC on Tumor Size Measurements of Invasive Breast Cancers
Get Radiology Tree app to read full this article<
Table 3
Comparison of the Differences Between Measured Tumor Size with Imaging and Histopathology
Tumor Type Ultrasound CESM Mean (mm) LOA (mm) Mean (mm) LOA (mm) Invasive cancer ( n = 7) −1.571 −19.48 to 14.4 −0.714 −11.15 to 9.72 Invasive cancer + EIC ( n = 13) −2.538 −12.82 to 9.67 0.846 −22.06 to 23.75 Total ( n = 20) −2.2 −17.1 to 12.7 0.3 −18.88 to 19.48
CESM, contrast-enhanced spectral mammography; EIC, extensive in situ component; LOA, limits of agreement.
t Test showed no significant under- or over-estimation of tumor size ( P < .05).
Get Radiology Tree app to read full this article<
Discussion
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
Get Radiology Tree app to read full this article<
References
1. Dromain C., Balleyguier C., Adler G., et. al.: Contrast-enhanced digital mammography. Eur J Radiol 2009; 69: pp. 34-42.
2. Lobbes M.B., Smidt M.L., Houwers J., et. al.: Contrast enhanced mammography: techniques, current results, and potential indications. Clin Radiol 2013; 68: pp. 935-944.
3. Dromain C., Thibault F., Muller S., et. al.: Dual-energy contrast-enhanced digital mammography: initial clinical results. Eur Radiol 2011; 21: pp. 565-574.
4. Jochelson M.S., Dershaw D.D., Sung J.S., et. al.: Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013; 266: pp. 743-751.
5. Fallenberg E.M., Dromain C., Diekmann F., et. al.: Contrast-enhanced spectral mammography versus MRI: initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol 2014; 24: pp. 256-264.
6. Litiere S., Werutsky G., Fentiman I.S., et. al.: Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol 2012; 13: pp. 412-419.
7. Pengel K.E., Loo C.E., Teertstra H.J., et. al.: The impact of preoperative MRI on breast-conserving surgery of invasive cancer: a comparative cohort study. Breast Cancer Res Treat 2009; 116: pp. 161-169.
8. Tendulkar R.D., Chellman-Jeffers M., Rybicki L.A., et. al.: Preoperative breast magnetic resonance imaging in early breast cancer: implications for partial breast irradiation. Cancer 2009; 115: pp. 1621-1630.
9. Uematsu T., Kasami M., Watanabe J.: Does the degree of background enhancement in breast MRI affect the detection and staging of breast cancer?. Eur Radiol 2011; 21: pp. 2261-2267.
10. Hieken T.J., Harrison J., Herreros J., et. al.: Correlating sonography, mammography, and pathology in the assessment of breast cancer size. Am J Surg 2001; 182: pp. 351-354.
11. Finlayson C.A., MacDermott T.A.: Ultrasound can estimate the pathologic size of infiltrating ductal carcinoma. Arch Surg 2000; 135: pp. 158-159.
12. Pritt B., Ashikaga T., Oppenheimer R.G., et. al.: Influence of breast cancer histology on the relationship between ultrasound and pathology tumor size measurements. Mod Pathol 2004; 17: pp. 905-910.
13. Gruber I.V., Rueckert M., Kagan K.O., et. al.: Measurement of tumour size with mammography, sonography and magnetic resonance imaging as compared to histological tumour size in primary breast cancer. BMC Cancer 2013; 13: pp. 328.
14. Uematsu T., Yuen S., Kasami M., et. al.: Comparison of magnetic resonance imaging, multidetector row computed tomography, ultrasonography, and mammography for tumor extension of breast cancer. Breast Cancer Res Treat 2008; 112: pp. 461-474.
15. Lobbes M.B., Nelemans P.J.: Good correlation does not automatically imply good agreement: the trouble with comparing tumour size by breast MRI versus histopathology. Eur J Radiol 2013; 82: pp. e906-e907.
16. Jiang Y.Z., Xia C., Peng W.T., et. al.: Preoperative measurement of breast cancer overestimates tumor size compared to pathological measurement. PLoS ONE 2014; 9: pp. e86676.
17. Schouten van der Velden A.P., Boetes C., Bult P., et. al.: The value of magnetic resonance imaging in diagnosis and size assessment of in situ and small invasive breast carcinoma. Am J Surg 2006; 192: pp. 172-178.
18. Grimsby G.M., Gray R., Dueck A., et. al.: Is there concordance of invasive breast cancer pathologic tumor size with magnetic resonance imaging?. Am J Surg 2009; 198: pp. 500-504.
19. Lobbes M.B., Lalji U., Houwers J., et. al.: Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur Radiol 2014; 24: pp. 1668-1676.
20. Fasching P.A., Heusinger K., Loehberg C.R., et. al.: Influence of mammographic density on the diagnostic accuracy of tumor size assessment and association with breast cancer tumor characteristics. Eur J Radiol 2006; 60: pp. 398-404.
21. Guinebretiere J.M., Le Monique G., Gavoille A., et. al.: Angiogenesis and risk of breast cancer in women with fibrocystic disease. J Natl Cancer Inst 1994; 86: pp. 635-636.